ABSTRACT
Pulmonary edema is a very prevalent condition, especially in the intensive care setting, and can have different origins and be caused by different mechanisms. Imaging tests, particularly computed tomography, are sometimes used in the diagnostic investigation of these patients, and may even help to elucidate the etiology. Therefore, it is essential that radiologists understand the dynamic nature of the changes associated with pulmonary edema and be able to recognize diagnostic clues that indicate possible etiologies, in addition to understanding the underlying mechanisms. This illustrated essay provides a concise review of the pathophysiology of pulmonary edema and highlights its main imaging findings, focusing on the clinical features that help to refine the etiological diagnosis.
Keywords:
Diagnostic imaging; Chest radiology; Pulmonary edema.
RESUMO
O edema pulmonar é uma condição bastante prevalente, sobretudo no contexto de terapia intensiva, ocasionado por diferentes origens e decorrente de mecanismos diversos. Por vezes, os exames de imagem, em particular a tomografia computadorizada, são utilizados na investigação diagnóstica desses pacientes, podendo auxiliar, inclusive, na elucidação etiológica. Portanto, é essencial que os radiologistas entendam a natureza dinâmica das alterações associadas ao edema pulmonar e sejam capazes de reconhecer pistas diagnósticas que indiquem possíveis etiologias, além de compreender os mecanismos subjacentes. Este ensaio ilustrado oferece uma revisão concisa da fisiopatologia do edema pulmonar e destaca seus principais achados em imagens, enfocando as características clínicas que ajudam a refinar o diagnóstico etiológico.
Palavras-chave:
Diagnóstico por imagem; Radiologia do tórax; Edema pulmonar.
INTRODUCTION
Pulmonary edema can be defined as an abnormal accumulation of fluid in the extravascular space within the interstitium and within alveoli(1). It is the result of an imbalance in Starling forces that is not fully corrected by physiological responses(1,2), which include increased lymphatic capacity and decreased interstitial oncotic pressure via the semipermeable barrier known as the alveolar-capillary membrane (Figure 1).
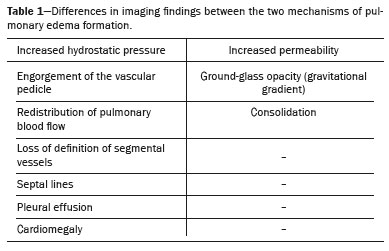
The Starling force imbalance
(2) explains the origin of the three main mechanisms of pulmonary edema formation, which facilitate understanding of the various chest imaging findings (Table 1). First, there is increased hydrostatic pressure
(1), which leads to a pressure imbalance when hydrostatic pressure exceeds oncotic pressure and fluid accumulates in the lung interstitium, potentially reaching the alveoli. The second mechanism is increased permeability
(1), which occurs when a significant inflammatory process causes damage to the capillary endothelium and alveolar epithelium. Finally, there is the mixed mechanism
(1), which is a combination of increased hydrostatic pressure and increased permeability
(1).
PULMONARY EDEMA DUE TO PRESSURE IMBALANCEPulmonary edema associated with altered hydrostatic pressure most often results from increased hydrostatic pressure and is primarily caused by heart failure
(2–5) or fluid overload
(1). It should be noted, however, that there are also cases of negative pressure edema, in which vascular pressure is normal but there is a greater reduction in alveolar pressure, generating a pressure differential that results in fluid migration into the alveolar space. Table 1 shows the imaging findings that corroborate this mechanism
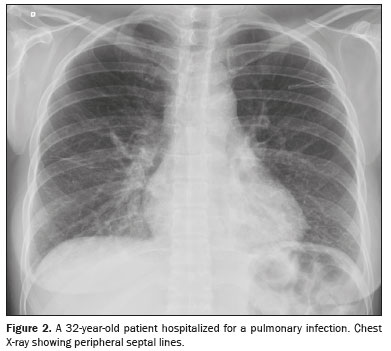
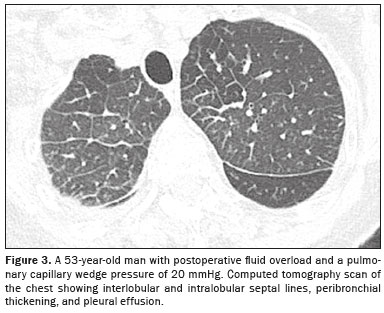
(1,6): engorgement of the vascular pedicle with enlargement of the upper portion of the mediastinum—above the aortic arch; cephalization, with redistribution of blood to the upper lobes; engorgement of the bronchovascular bundle caused by increased interstitial thickness around the walls of a bronchus or bronchiole, which, on tomography, appears similar to bronchial wall thickening without luminal narrowing; septal lines
(3), previously known as Kerley B lines, which reflect congestion and engorgement of the veins and lymphatic vessels in the interlobular septa, as seen on chest X-rays (Figures 2 and 3); increased density or ground-glass opacities; and, more commonly, pleural effusion and signs of cardiomegaly
(2,3).
ACUTE LUNG EDEMAIn cases of a rapid increase in pulmonary pressure, there can be extravasation of fluid into the alveolar space, which tends to occur more commonly in the central regions of the lungs, where the pressure is higher, generating the classic appearance of ground-glass opacities and consolidations in a bat-wing pattern
(1), in which the periphery is unaffected (Figure 4).
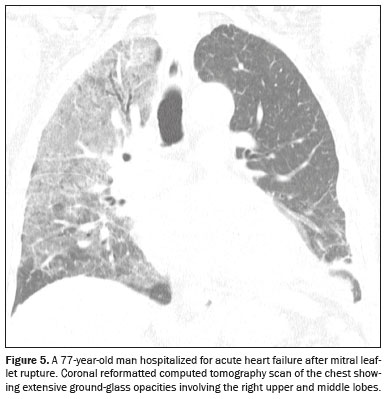
There is another atypical form related to increased hydrostatic pressure: asymmetric pulmonary edema that occurs as a consequence of acute mitral insufficiency
(3), resulting in a sudden increase (to 10–15 mmHg) in the left atrial pressure
(1), with the regurgitant jet most frequently directed toward the ostium of the right superior pulmonary vein, leading to unilateral edema, which usually affects the upper lobe of the right lung
(1), as illustrated in Figure 5.
NEGATIVE-PRESSURE (POSTOBSTRUCTIVE) PULMONARY EDEMANegative-pressure pulmonary edema, also known as postobstructive pulmonary edema, occurs after sudden upper airway obstruction in cases of laryngospasm, epiglottitis, strangulation, recent extubation, or seizure. The patient performs forced inspirations, generating abnormally negative intrathoracic pressures (reaching −50 to −100 cmH
2O), thus increasing venous return to the right heart and elevating hydrostatic pressure in the pulmonary capillaries, favoring fluid extravasation into the interstitium and alveoli, as well as the formation of edema. In such patients, tomography reveals ground-glass opacities, with or without consolidations, predominantly in the central regions of the lungs, with no pleural effusion or smooth septal thickening outside the affected areas (Figure 6).As described by Cascade et al.
(4), negative-pressure pulmonary edema resolves rapidly after airway clearance and adequate ventilatory support.
PULMONARY EDEMA DUE TO INCREASED VASCULAR PERMEABILITYEdema due to increased vascular permeability arises from changes in the permeability of the alveolar–capillary membrane, and there may or may not be damage to the alveolar epithelium
(1–3). The main clinical entity associated with this histological pattern is diffuse alveolar damage
(2,5), which increases vascular permeability, causing direct damage to the alveolus. The differential diagnosis should include other etiologies, although only those that do not cause significant alveolar damage, such as high-altitude pulmonary edema
(1).
DIFFUSE ALVEOLAR DAMAGEDiffuse alveolar damage reflects an alteration of the biochemical balance, with an excess of cytokines and interleukins, which increase the permeability of the vascular endothelial plasma membrane, with direct damage to the membrane by inflammatory cells
(1,2). The most classic imaging findings in the acute phase include ground-glass opacities and consolidations, typically with a gravitational gradient
(1), with denser opacities being noted in the dependent regions of the lung (Figure 7). Some cases progress to fibrosis, typically in the most anterior/non-dependent regions, through a barotrauma/volutrauma mechanism.
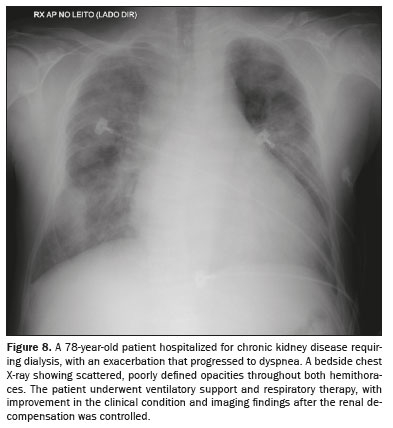
UREMIC PULMONARY EDEMAPulmonary edema of uremic origin is the result of changes in the pulmonary intravascular Starling forces due to increased permeability of the pulmonary capillary membrane, due to a high protein content in the pulmonary edema fluid. On imaging (Figure 8), sparse foci pulmonary congestion are quite often observed, with an appearance that can resemble bat-wing edema
(7,8).
MIXED-MECHANISM PULMONARY EDEMAPulmonary edema due to a mixed mechanism involves changes in hydrostatic pressure and in vascular permeability
(1,2), with the main representatives being neurogenic pulmonary edema, pulmonary edema due to reperfusion, pulmonary edema after lung transplantation, pulmonary edema due to lung reexpansion, and postpneumonectomy pulmonary edema
(1,2).
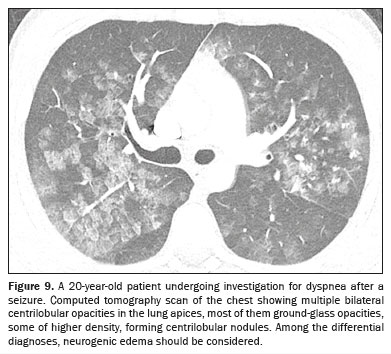
NEUROGENIC PULMONARY EDEMAThe diagnosis of neurogenic pulmonary edema requires correlation with the clinical history, given that it manifests as acute respiratory distress secondary to severe neurological events
(9), such as head trauma, subarachnoid hemorrhage, and seizure
(1,9). It is believed to involve autonomic dysfunction with systemic vasoconstriction and release of inflammatory mediators, causing pulmonary hypertension and increasing capillary permeability
(1). Imaging shows bilateral consolidations, predominantly at the lung apices (Figure 9), with a heterogeneous pattern. With appropriate treatment of the neurological cause, the thoracic findings usually resolve within 48 h
(1).
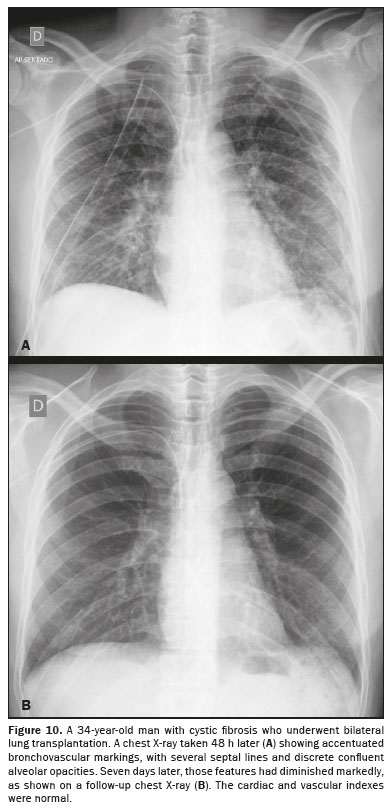
PULMONARY EDEMA AFTER LUNG TRANSPLANTATIONPulmonary edema after lung transplantation involves the permeability mechanism, due to the hypoxia process in the donor lung, which generates ischemia and a loss of surfactant, as well as interrupting surgical lymphatic drainage and surgical denervation
(1). The most characteristic imaging findings are perihilar ground-glass opacities, thickening of the peribronchial interstitium, and alveolar hyperdensity in the lower lobes
(1,2), as shown in Figure 10, all of which appear 1–7 days after lung transplantation
(1).
PULMONARY EDEMA AFTER NONFATAL DROWNINGAnother uncommon etiology of pulmonary edema is an episode of inhalation, or aspiration, of water (typically during nonfatal drowning) within the preceding 24 h. There are two mechanisms of such edema: negative pressure due to laryngeal spasm; and increased permeability due to alveolar damage triggered by aspirated water coming into contact with the alveoli. As illustrated in Figures 11 and 12, the imaging findings of pulmonary edema after nonfatal drowning are similar to those of other causes of noncardiogenic edema, usually without cardiomegaly or pleural effusion, with a history of nonfatal drowning being essential
(1).
REEXPANSION PULMONARY EDEMAReexpansion pulmonary edema is associated with a history of lung reexpansion after thoracentesis. This type of pulmonary edema typically occurs, via a mixed mechanism, 48 h after the procedure, resolving by postprocedure day 7
(1). Typical radiological manifestations include perihilar ground-glass opacities and small central consolidations on the drained, reexpanded side, as well as peribronchial thickening. Common findings include areas of ground-glass opacity and even consolidation
(10,11), together with thickening of the bronchovascular bundles and interlobular septal thickening
(1), as illustrated in Figure 13.
HIGH-ALTITUDE PULMONARY EDEMAThe epidemiological history also plays a very important role in the diagnosis of high-altitude pulmonary edema, given that the condition is directly related to exposure to low oxygenation, typically occurring within three days after an ascent to an altitude above 3,000 m
(1). The mechanism is classified as mixed, due to transient sympathetic discharge causing pulmonary vasoconstriction that leads to pressure imbalance and changes in the transmembrane potential
(1), as depicted in Figure 14.
LUNG ULTRASOUNDLung ultrasound, a noninvasive, radiation-free examination, is especially useful in intensive care units because it allows rapid differentiation among the various types of pulmonary edema. The presence of septal lines—reverberation artifacts originating from the hyperechoic pleural line—allows for semiquantitative estimation of extravascular lung water content, ranging from normal to indicative of severe edema
(12,13). Bilateral pleural-line abnormalities, such as thickening, irregularity, or fragmentation, are suggestive of noncardiogenic edema
(14). Surface wave elastography can be employed to assess the elastic properties of lung tissue, contributing to the characterization of edema
(13).
CONCLUSIONPulmonary edema, which reflects the leakage of fluid into the interstitium and alveolar spaces, has several underlying mechanisms. Imaging, combined with clinical history, can lead to a diagnosis in challenging cases and can allow timely follow-up of affected patients. Therefore, radiologists must understand the mechanisms and key imaging aspects involved.
REFERENCES1. Gluecker T, Capasso P, Schnyder P, et al. Clinical and radiologic features of pulmonary edema. Radiographics. 1999;19:1507–33.
2. Barile M. Pulmonary edema: a pictorial review of imaging manifestations and current understanding of mechanisms of disease. Eur J Radiol Open. 2020;7:100274.
3. Swensen SJ, Aughenbaugh GL, Douglas WW, et al. High-resolution CT of the lungs: findings in various pulmonary diseases. AJR Am J Roentgenol. 1992;158:971–9.
4. Cascade PN, Alexander GD, Mackie DS. Negative-pressure pulmonary edema after endotracheal intubation. Radiology. 1993;186:671–5.
5. Malek R, Soufi S. Pulmonary edema. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan.
6. Assaad S, Kratzert WB, Shelley B, et al. Assessment of pulmonary edema: principles and practice. J Cardiothorac Vasc Anesth. 2018;32:901–14.
7. Rackow E, Fein IA, Sprung C, et al. Uremic pulmonary edema. Am J Med. 1978;64:1084–8.
8. Hublitz UF, Shapiro JH. Atypical pulmonary patterns of congestive failure in chronic lung disease. The influence of pre-existing disease on the appearance and distribution of pulmonary edema. Radiology. 1969;93:995–1006.
9. Al-Dhahir MA, Das JM, Hall WA, et al. Neurogenic pulmonary edema. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan.
10. Baik JH, Ahn MI, Park YH, et al. High-resolution CT findings of re-expansion pulmonary edema. Korean J Radiol. 2010;11:164–8.
11. Corcoran JP, Psallidas I, Barker G, et al. Reexpansion pulmonary edema following local anesthetic thoracoscopy: correlation and evolution of radiographic and ultrasonographic findings. Chest. 2014;146:e34–e37.
12. Picano E, Pellikka PA. Ultrasound of extravascular lung water: a new standard for pulmonary congestion. Eur Heart J. 2016;37:2097–104.
13. Wiley BM, Zhou B, Pandompatam G, et al. Lung ultrasound surface wave elastography for assessing patients with pulmonary edema. IEEE Trans Biomed Eng. 2021;68:3417–23.
14. Heldeweg MLA, Smit MR, Kramer-Elliott SR, et al. Lung ultrasound signs to diagnose and discriminate interstitial syndromes in ICU patients: a diagnostic accuracy study in two cohorts. Crit Care Med. 2022;50:1607–17.
1. Hospital Israelita Albert Einstein, São Paulo, SP, Brazil
a.
https://orcid.org/0009-0005-6659-0730 b.
http://orcid.org/0000-0002-0233-0041 c.
https://orcid.org/0009-0006-5958-8461 d.
https://orcid.org/0009-0003-3567-6633 e.
https://orcid.org/0000-0002-6152-3556 f.
https://orcid.org/0000-0002-8571-5667 g.
https://orcid.org/0000-0003-4277-9131 h.
https://orcid.org/0000-0003-3748-5649 i.
https://orcid.org/0000-0002-4193-7647Correspondence: Dr. Matheus Marcelino Dias
Hospital Israelita Albert Einstein
Avenida Albert Einstein, 627, Jardim Leonor
São Paulo, SP, Brazil, 05652-900
Email:
dr.matheusmdias@gmail.com
Received in
December 1 2024.
Accepted em
May 30 2025.
Publish in
September 22 2025.

 |
|