Radiologia Brasileira - Publicação Científica Oficial do Colégio Brasileiro de Radiologia
AMB - Associação Médica Brasileira CNA - Comissão Nacional de Acreditação
 Vol. 42 nº 3 - May / June of 2009
Vol. 42 nº 3 - May / June of 2009
|
ORIGINAL ARTICLE
|
|
Proton spectroscopy study of the masseter in patients with systemic sclerosis |
|
|
Autho(rs): Marcelo Marcucci, Nitamar Abdala |
|
|
Keywords: Masseter, Systemic sclerosis, Proton spectroscopy |
|
|
Abstract:
IPhD, Supervisor of the Technical Team of the Division of Stomatology and Oromaxillofacial Surgery, Hospital Heliópolis - SUS, São Paulo, SP, Brazil
INTRODUCTION Systemic sclerosis is a chronic autoimmune inflammatory disease of unknown etiology, characterized by excessive deposition of collagen and glycosaminoglycans in the connective tissue of the skin and internal organs(1-3). This disease has a low incidence disease, affecting 19 individuals per million inhabitants, with prevalence in women (3:1). The most affected age group is between the third and fifth decade of life(2). Raynaud's phenomenon is usually the first clinical manifestation among several clinical findings such as skin thickening(4), esophageal dysmotility, restrictive pulmonary disease(5-7), pulmonary hypertension, arthralgias, myopathies, myocardiopathy, and progressive renal failure(1-3,8). Musculoskeletal system involvement is one of the most relevant causes of disability in systemic sclerosis(9). Frequently, erosion of the intermediate and terminal portions of the phalanges (acroosteolysis) is observed, secondary to the involvement of the fingertips skin. Less frequently, the second and fifth ribs, cervical vertebrae, the distal portion of the clavicle, radius and ulna(10,11) are affected. Systemic sclerosis presents characteristic alterations in the maxillomandibular complex, such as thickening of periodontal ligament and areas of osteolysis in the mandible as described by Taveras(12) in 1959, coinciding with the zones of insertion of the lateral pterygoid, temporal and mainly the masseter muscles. Only a few studies have evaluated the involvement of the masseter muscle in systemic sclerosis as well as the possible association between this involvement and areas of mandibular osteolysis by means of electroneuromyography(13) and magnetic resonance imaging (MRI)(14). Based on studies on mandible microvascularization, Ramón et al.(15) have proposed that osteolysis originates from muscle ischemia resulting from microvasculopathy typical of systemic sclerosis. Muscular involvement in systemic sclerosis is probably caused by ischemia, which would lead to an insufficient oxygen and nutrient supply, inflammation, or to the sclerotic process itself. Thus, myopathy would be a primary process of the disease, and not necessarily resulting from the superjacent skin involvement(16,17). Studies about capillary and arteriolar circulation have shown thickening of the lumen, flow reduction, stenosis and alterations of the capillary architecture(18). The present study was aimed at evaluating the creatine, choline, lipid and lactate concentrations in the masseter muscle of patients with systemic sclerosis, by means of hydrogen spectroscopy, and correlating the results with the presence of mandibular osteolysis.
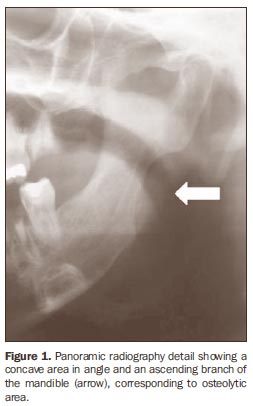
MATERIALS AND METHODS The present study included 25 male and female individuals, 15 of them (mean age = 43.72 ± 7.59 years) with diffuse systemic sclerosis, and 10 (mean age = 31.82 ± 12.64 years) without the disease forming a control group. Patients with limited systemic sclerosis or in association with other rheumatic diseases were excluded. The 15 patients with systemic sclerosis were divided into two groups - group I, with seven patients with systemic sclerosis and mandibular osteolysis (Figure 1), and group II, with eight patients with systemic sclerosis without mandibular osteolysis - and the group III included the ten healthy individuals constituting the control group. The present study was approved by the Committee for Ethics in Research, and all the individuals signed a term of free and informed consent.
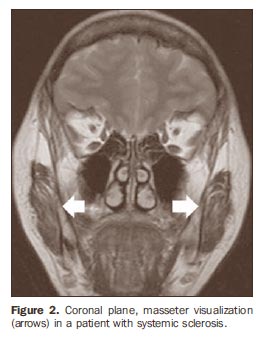
All the individuals were submitted to bilateral MRI study of the masseter (Figure 2) in a 1.5 T Sonata® (Siemens Medical Systems; Erlangen, Germany) equipment with 40 mT gradient. Turbo spin echo (TSE) T2-weighted sequences were acquired with relaxation in the coronal plane, 2810 ms repetition time (TR), 84 ms echo time (TE), number of excitations (NEX) 2, field of view (FOV) of 230 mm and 5 mm interval. T1-weighted sequences were acquired with relaxation in the axial plane (479 ms TR; 13 ms TE; NEX 2; FOV 230 mm; 5 mm interval) with and without fat suppression (420 ms TR; 13 ms TE; NEX 2; FOV 230 mm; 5 mm interval). Total acquisition time was 15 minutes, and total examination time was 25 minutes.
Magnetic resonance spectroscopy (MRS) with PRESS sequence was performed with a 3D-positioned single voxel. The region of interest (ROI) was represented by a cubic volume visually positioned at the central region of the muscle that anatomically is the site with largest tissue mass (Figure 3). Thus, the frequencies reading was focused exclusively on the masseter muscle, without interferences from surrounding tissues. Values for both masseters were obtained in groups I and II, while in group III, the acquisition was unilateral. Only the metabolites related to the muscle tissue were recorded with the respective frequency scales, in parts per million: creatine (3.14), choline (3.30), lactate (1.14) and lipid (1.42). The variance analysis (ANOVA) was utilized for statistical analysis of the metabolites creatine, choline, lipid and lactate ratios, followed by the Bonferroni or Tamhane multiple comparisons, as necessary, besides Brown-Forsythe test for equality of groups variance corrections. The adopted rejection level for null hypothesis was 0.10 (10%). The software SPSS 11.5 was utilized for statistical analysis.
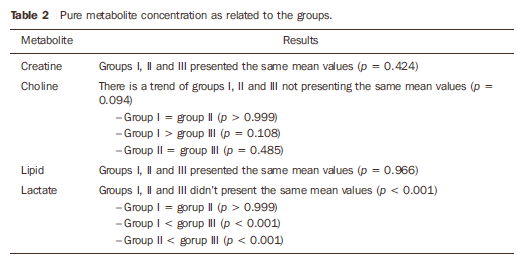
RESULTS The values for pure metabolites are listed on Table 1. The analysis of pure metabolites that can be observed on Table 2, demonstrated no significant difference regarding creatine and lipid among the three groups. Regarding choline, no significant difference was observed between patients with and without osteolysis. The lactate concentration was lower in patients with systemic sclerosis, with or without osteolysis, as compared with the healthy individuals.
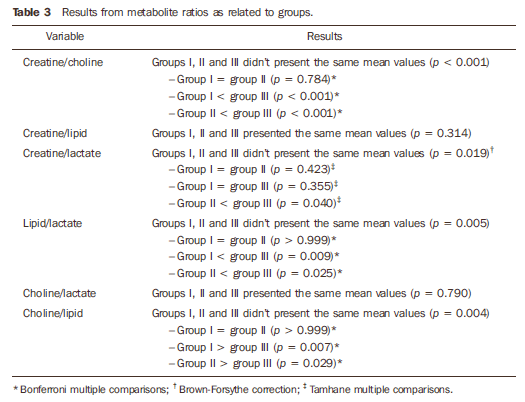
The analysis of metabolite ratios demonstrated differences among the groups for creatine/choline, creatine/lactate, lipid/lactate and choline/lipid (Table 3).
DISCUSSION MRS was developed early in the nineties, as a noninvasive method for evaluating metabolites concentration in different tissues, such as the brain, heart and muscles(19). This method has been of invaluable help for studying the facial muscles metabolism, considering that conventional methods required biopsies, with great limitations for this type of study(20). Proton magnetic resonance spectroscopy (1H-MRS) was chosen considering that hydrogen is the most abundant atom in the organism, with higher sensitivity, easy performance and interpretation, besides the capacity to investigate a higher number of metabolites as compared with phosphorus spectroscopy (31P-MRS)(21,22). In the present study, creatine was the evaluated component of the energy chain. It acts as a creatine kinase substrate transferring the phosphate group of the triphosphate adenosine molecule to creatine for the production of phosphocreatine. Phosphocreatine, on its turn, is the storing compound utilized for energy production during the muscular effort(23). Creatine values were statistically equivalent in the three groups, but a previous study with 1H-MRS found a higher creatine concentration in the masseter muscle of patients with temporomandibular joint disorder, indicating higher intensity and/or frequency of biochemical reactions necessary for sustaining the muscular function(24). Creatine levels remain stable in the brain tissue in several diseases(21,23), however this assertion is not valid for the musculoskeletal system, considering that some studies about the energy metabolism in rheumatic diseases have demonstrated variable results. By submitting lower limb muscles to 31P-MRS, some studies have observed increased inorganic phosphate/phosphocreatine levels in patients with systemic sclerosis and polymyositis(15,25), which indicates the presence of ischemic muscle disease. On the other hand, studies with 1H-MRS have shown a decrease in creatine concentration in the calf of patients with eosinophilia-myalgia syndrome(26) and in patients with Duchenne muscular dystrophy(27). As regards to the presence of lactate, the patients with systemic sclerosis presented lower concentration than the healthy individuals. On the other hand, in the present study no difference was observed between patients with and without osteolysis. An in vitro study about muscular metabolism in Duchenne muscular dystrophy has demonstrated a decrease in lactate levels in healthy individuals: the decrease in lactate concentration indicates a reduction of glycolytic activity, or even a decrease in lactate concentration in the muscle(27). In the present study, the interpretation of this finding can be based on the assumption that the vascular phenomena related to systemic sclerosis are not reducing the aerobic capacity of the masseter because of the absence of anaerobic glycolysis (lactic acid system), in the same way that there was no change in the energy demand (creatine). So, these phenomena would not significantly act on the masseter, or would act in a partial way, so that the extensive vascular network of the masseter would, in a way, be meeting its oxidative energy needs. Then, both biochemical factors do not appear to significantly contribute to the osteolysis genesis, as observed in the present study. In the same way as creatine, no significant difference in lipid levels was observed among the three groups. Increased lipid concentration is related to cell degradation and necrosis(21), and this increase was observed in patients with temporomandibular joint disorder(24). Choline was the only metabolite that showed increased level in healthy individuals. The choline level increase reflects a process of cellular proliferation and evidence of cell membrane synthesis phenomena like those observed in neoplastic diseases, being increased in relation to healthy muscles(21,28). On the other hand, a decrease in choline levels may demonstrate abnormalities in the membrane, as observed in patients with Duchenne muscular dystrophy(27). Increased choline levels may be related to cell proliferation and/or increased density, and besides that, inflammatory processes may also demonstrate peaks of this metabolite resulting from the presence of a large population of inflammatory cells(28). Thus, the higher choline concentration in patients with osteolysis may be related to pathophysiological mechanisms that affect the fibroblast in systemic sclerosis(29) and to the growth of conjunctive tissue in the epimysium and perimysium(1). Additionally, it was observed that, in systemic sclerosis, one observed that that in systemic sclerosis the tissue hypoxia induces neoangiogenesis and that there is an intense proliferation of the vascular intima layer(30). Therefore, increased choline levels may be a product of the above mentioned phenomena, as well as expressing an inflammatory activity in the masseter muscle, with these factors as possible participants in the genesis of osteolysis. The metabolites ratios were analyzed to evaluate their activity as a whole. The creatine/lipid and choline/lactate ratios were statistically equivalent in the three groups. On the other hand, the other ratios presented variances suggesting significance. Previous studies have showed that choline/ lipid and creatine/lipid ratios are decreased in patients with polymyositis(26), while in the present study, an increase was observed in the choline/lipid ratio and absence of variation in the creatine/lipid ratio in patients with systemic sclerosis. Patients with systemic sclerosis also presented lower creatine/choline and lipid/lactate ratios when compared with healthy individuals. As regards creatine/lactate ratio, only the patients without osteolysis showed a decrease in relation to healthy individuals. Findings at 1H-MRS should be carefully interpreted, as they may be a consequence of interaction of factors such as the disease activity degree, level of muscle involvement and may even be influenced by medicines such as corticosteroids utilized in the management of the disease(22). On the other hand, this method demonstrates biochemical alterations even in patients with no clinical or morphological evidence of muscle alterations(25).
CONCLUSIONS Lower lactate levels were observed in the masseter muscle of patients with systemic sclerosis as compared with healthy individuals, while choline levels tend to be increased in patients with osteolysis. Creatine/choline, creatine/lactate, lipid/lactate and choline/lipid ratios showed a trend towards difference among the groups in the present study. Further studies are necessary to understand the role played by the masseter in the development of mandibular osteolysis.
REFERENCES 1. Kayser C, Andrade LEC. Esclerose sistêmica. In: Sato E. Guias de medicina ambulatorial e hospitalar - reumatologia. São Paulo: Ed. Manole; 2004. p. 111-20. [ ] 2. Mayes MD, Lacey JV Jr, Beebe-Dimmer J, et al. Prevalence, incidence, survival, and disease characteristics of systemic sclerosis in a large US population. Arthritis Rheum. 2003;48:2246-55. [ ] 3. Badea I, Taylor M, Rosenberg A, et al. Pathogenesis and therapeutic approaches for improved topical treatment in localized scleroderma and systemic sclerosis. Rheumatology (Oxford). 2009;48:213-21. [ ] 4. Bouer M, Chammas MC, Messina MCL, et al. Correlação clínica e ultra-sonográfica na esclerodermia localizada cutânea. Radiol Bras. 2008;41: 87-91. [ ] 5. Azevedo ABC, Guimarães SMM, Tavares Jr WC, et al. Avaliação da tomografia de alta resolução versus radiografia de tórax na doença intersticial pulmonar na esclerose sistêmica. Radiol Bras. 2005;38:95-9. [ ] 6. Gasparetto EL, Pimenta R, Inoue C, et al. Esclerose sistêmica progressiva: aspectos na tomografia computadorizada de alta resolução. Radiol Bras. 2005;38:329-32. [ ] 7. Santos MK, Faria FB, Trad CS. Comprometimento pulmonar na esclerose sistêmica: revisão de casos. Radiol Bras. 2006;39:181-4. [ ] 8. Avouac J, Kowal-Bielecka O, Landewé R, et al. European League Against Rheumatism (EULAR) Scleroderma Trial and Research group (EUSTAR) recommendations for the treatment of systemic sclerosis: methods of elaboration and results of systematic literature research. Ann Rheum Dis. 2009;68:629-34. [ ] 9. Pope JE. Musculoskeletal involvement in scleroderma. Rheum Dis Clin North Am. 2003;29:391-408. [ ] 10. Bassett LW, Blocka KLN, Furst DE, et al. Skeletal findings in progressive systemic sclerosis (scleroderma). AJR Am J Roentgenol. 1981;136: 1121-6. [ ] 11. Rosa ACF, Costa EN, Machado MM, et al. Calcinose peritendínea do tendão calcâneo associada a dermatomiosite: correlação entre radiografia convencional, ultra-sonografia, ressonância magnética e macroscopia cirúrgica. Radiol Bras. 2006; 39:75-8. [ ] 12. Taveras JM. The interpretation of radiographs. In: Schwartz L, editor. Disorders of the temporomandibular joint. Philadelphia: Saunders; 1959. p. 154-62. [ ] 13. Pogrel MA. Unilateral osteolysis of the mandibular angle and coronoid process in scleroderma. Int J Oral Maxillofac Surg. 1988;17:155-6. [ ] 14. Ruprecht A, Dolan K, Lilly GE. Osteolysis of the mandible associated with progressive systemic sclerosis. Dentomaxillofac Radiol. 1990;19:31-3. [ ] 15. Ramón Y, Samra H, Oberman M. Mandibular condylosis and apertognathia as presenting symptoms in progressive systemic sclerosis (scleroderma). Pattern of mandibular bony lesions and atrophy of masticatory muscles in PSS, presumably caused by affected muscular arteries. Oral Surg Oral Med Oral Pathol. 1987;63:269-74. [ ] 16. King LE Jr, Olsen NJ, Puett D, et al. Quantitative evaluation of muscle weakness in scleroderma patients using magnetic resonance imaging and spectroscopy. Arch Dermatol. 1993;129:246-7. [ ] 17. Olsen NJ, King LE Jr, Park JH. Muscle abnormalities in scleroderma. Rheum Dis Clin North Am. 1996;22:783-96. [ ] 18. Schmidt WA, Wernicke D, Kiefer E, et al. Colour duplex sonography of finger arteries in vasculitis and in systemic sclerosis. Ann Rheum Dis. 2006;65:265-7. [ ] 19. Ross B, Kreis R, Ernst T. Clinical tools for the 90s: magnetic resonance spectroscopy and metabolite imaging. Eur J Radiol. 1992;14:128-40. [ ] 20. Al-Farra ET, Vandenborne K, Swift A, et al. Magnetic resonance spectroscopy of the masseter muscle in different facial morphological patterns. Am J Orthod Dentofacial Orthop. 2001;120:427-34. [ ] 21. Ramin SL, Tognola WA, Spotti AR. Proton magnetic resonance spectroscopy: clinical application in patients with brain lesions. São Paulo Med J. 2003;121:254-9. [ ] 22. Chung YL, Smith EC, Williams SCR, et al. In vivo proton magnetic resonance spectroscopy in polymyositis and dermatomyositis: a preliminary study. Eur J Med Res. 1997;2:483-7. [ ] 23. Miller BL. A review of chemical issues in H NMR spectroscopy: N-acetyl L-aspartate, creatine and choline. NMR Biomed. 1991;4:47-52. [ ] 24. Nasri LFG. Análise comparativa entre os achados de ressonância magnética e de eletromiografia do músculo facial masseter, em indivíduos com e sem disfunção têmporo-mandibular [tese de doutorado]. São Paulo: Universidade Federal de São Paulo; 2005. [ ] 25. Doornbos J, Luyten PR, Janssen M, et al. P-31 MR spectroscopy of skeletal and cardiac muscle metabolism in patients with systemic sclerosis: a multiple case study. J Magn Reson Imaging. 1994;4:165-8. [ ] 26. Schick F, Duda S, Dürk H, et al. Eosinophiliamyalgia syndrome: findings at MR imaging and proton spectroscopy of the lower leg. Magn Reson Imaging. 1994;12:513-22. [ ] 27. Sharma U, Atri S, Sharma MC, et al. Skeletal muscle metabolism in Duchenne muscular dystrophy (DMD): an in-vitro proton NMR spectroscopy study. Magn Reson Imaging. 2003;21:145-53. [ ] 28. Maheshwari SH, Mukherji SK, Neelon B, et al. The choline/creatine ratio in five benign neoplasms: comparison with squamous cell carcinoma by use of in vitro MR spectroscopy. AJNR Am J Neuroradiol. 2000;21:1930-5. [ ] 29. Finol HJ, Marquez A, Rivera H, et al. Ultrastructure of systemic sclerosis inflammatory myopathy. J Submicrosc Cytol Pathol. 1994;26:245-53. [ ] 30. Herron GS, Romero LI. Vascular abnormalities in scleroderma. Semin Cutan Med Surg. 1998;17: 12-7. [ ] Received November 18, 2008. Accepted after revision March 30, 2009. * Study developed at the Department of Imaging Diagnosis - Universidade Federal de São Paulo/Escola Paulista de Medicina (Unifesp/EPM), São Paulo, SP, Brazil. |
|
Av. Paulista, 37 - 7° andar - Conj. 71 - CEP 01311-902 - São Paulo - SP - Brazil - Phone: (11) 3372-4544 - Fax: (11) 3372-4554