Radiologia Brasileira - Publicação Científica Oficial do Colégio Brasileiro de Radiologia
AMB - Associação Médica Brasileira CNA - Comissão Nacional de Acreditação
 Vol. 43 nº 2 - Mar. / Apr. of 2010
Vol. 43 nº 2 - Mar. / Apr. of 2010
|
ORIGINAL ARTICLE
|
|
Evaluation of a portable device for vacuum-assisted biopsy of breast microcalcifications |
|
|
Autho(rs): Hélio Sebastião Amâncio de Camargo Júnior, Márcia Martos Amâncio de Camargo, Sandra Regina Campos Teixeira, Juliana Azevedo, Maurício de Souza Arruda |
|
|
Keywords: Breast biopsy, Vacuum-assisted biopsy, Mammotomy, Mammography, Microcalcifications, Vacora |
|
|
Abstract:
Hélio Sebastião Amâncio de Camargo JúniorI; Márcia Martos Amâncio de CamargoII; Sandra Regina Campos TeixeiraII; Juliana AzevedoII; Maurício de Souza ArrudaIII ITitle of Specialist in Radiodiagnosis and Mastology, Director for CDE Diagnóstico por Imagem, Campinas, SP, Brazil
INTRODUCTION Vacuum-assisted biopsy is a percutaneous (non-surgical) way to obtain breast tissue for histological analysis, thus being considered as a minimally invasive procedure. It can be performed under mammographic guidance (in this case requiring the use of stereotactic resources), sonographic guidance or magnetic resonance imaging guidance. It is the type of percutaneous biopsy that obtains the largest sampling of tissue(1), and studies have demonstrated that the adoption of such technique results in a lower underestimation rate in the diagnosis of breast microcalcifications as compared with simple stereotactic core biopsy (that is, non-vacuum-assisted biopsy), decreasing the need for surgical biopsies(2,3). The disadvantage of the vacuum-assisted biopsy is related to cost, which is considerably higher than that of simple core biopsy. Initially, there was a single sales representative of vacuum-assisted biopsy devices in Brazil, and the most common name attributed to the procedure in Portuguese, mamotomia, is actually a reference to the trademark of such device. Currently, other vacuum-assisted biopsy devices are available in Brazil. One of them is a handheld device (Figure 1) that does not require cables or a separate vacuum generating unit. This system has a considerably lower cost (approximately 75% lower), and for this reason it may be an advantageous alternative when the economic reality of our country is considered. The potential disadvantage of the method lies in the need of repeated probe insertions at each sample collection, which may increase the time required for the procedure and affect the method accuracy. Additionally, one might question whether the vacuum produced by such portable system has enough pressure to produce samples with diagnostic quality.
The present study was designed to test the performance of such equipment in microcalcifications biopsy concerning the appropriateness of tissue samples for histopathological analysis, and the possible inconveniences caused by the need of repeated probe insertions.
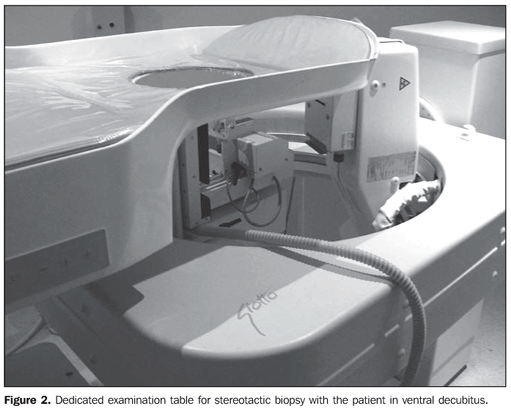
MATERIALS AND METHODS Vacuum assisted biopsies were performed with a Vacora® device (Bard Biopsy Systems; Temple, AZ, USA) in 35 consecutive patients presenting with BI-RADS® 4 or 5 microcalcifications between January and October 2008. Mean patients' age was 54 years. The exclusion criteria would be patients with allergy to anesthetics or those unable to remain still during the time required for the procedure, but no patient of the sample was excluded. All the procedures were performed by one of two practitioners, both of them with more than seven-year experience in breast radiology. Stereotactic guidance was utilized in all cases with a dedicated Giotto system (IMF; Bologne, Italy), with the patient positioned in ventral decubitus, the breast being approached though an aperture on the examination table (Figure 2). Local anesthesia (lidocaine), without vasoconstrictor on the skin, and with vasoconstrictor in the deep planes, was utilized. On average, 12 fragments were obtained in each collection cycle.
The device's conventional technique was utilized. The cannula is always positioned on the lesion central point, as determined by stereotaxy. Successive collections are then performed, in radial orientation at 30° steps, until a complete circumference is completed around the cannula. The device is removed for tissue sample retrieval after each collection, and is then reinserted in the same position. The change in orientation of the collection window of the cannula is selected on the device itself (Figure 3). The presently tested system is not equipped with a post-collection hematoma aspiration device.
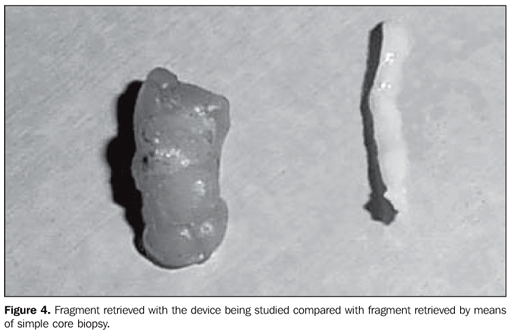
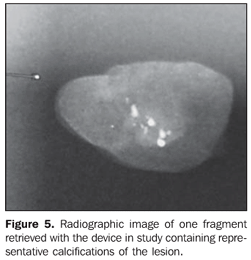
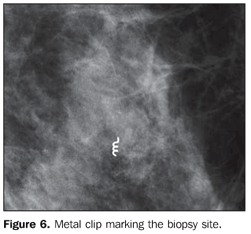
The following parameters were tested: sample appropriateness, number of collection cycles and technical difficulty in the repeated cannula insertion. The retrieved fragments (Figure 4) were radiographed (Figure 5). In the presence of representative calcifications, a metal clip was placed, marking the biopsy site (Figure 6), except in cases of very evident residual lesions that could be utilized as a biological marker in the case of re-intervention. In the absence of representative calcifications, the procedure was repeated until they were obtained.
Representative calcifications were so considered when at least five clustered calcifications were obtained, and such calcifications included some of the most suspicious for malignancy. After the procedure was completed, two mammographic views were performed to confirm the calcifications extraction and the clips positioning. In the cases diagnosed as benign, a six-month radiological follow-up was recommended.
RESULTS All the biopsies obtained calcifications representative of the radiological lesions. Anatomopathological studies revealed benign findings in 23 cases (typical hyperplasia in 5, psammomatous calcifications in 6, simple adenosis in 1, dystrophic calcifications in 2 and benign calcifications not specified in the anatomopathological reports in 3), ductal carcinoma in situ in 8 cases, 4 of them high-grade, invasive ductal carcinoma in 2 cases, both of them high-grade, and high risk lesion (atypical ductal hyperplasia) in 2 cases. Among the 8 cases of ductal carcinoma in situ, definitive surgery revealed invasive ductal carcinoma in 1 case. In the 2 cases of high-risk lesions, the patient was submitted to surgical biopsy, and the diagnosis was confirmed as benign. In 6 cases two collection cycles were required, and in the remaining 29 cases a single collection cycle was performed. Technical difficulties were not observed with the repeated probe insertions, as it was demonstrated that the stereotactic guides directed the probe exactly to the skin nick previously made, since the patient's breast remains fixed during the whole procedure. Lesions were completely removed in 8 cases and partial sampling occurred in 27 cases. Metal clips were utilized in 31 cases. No error was observed in the marker clips placement. Severe complications were not observed. Hematomas occurred in 15 cases (43%), 3 of them being large (9%). There was no need to drain such hematomas. On average, each collection cycle took eight minutes to be completed. With respect to pain, the procedure was well tolerated by all the patients. Occasionally, additional local anesthetic administration was required because of pain complaint during the procedure. The tested device does not allow the application of anesthetics directly through the biopsy cannula. Therefore, whenever necessary, the additional anesthetic application was performed by means of a needle inserted beside the probe, or by removing the cannula and inserting the needle in the same biopsy pathway.
DISCUSSION This study was aimed at testing the performance of a hand-held vacuum assisted device and has found results similar to those achieved with non portable equipment. The paradigm to be taken into consideration in the selection of a method for a given breast lesion biopsy should be the utilization of a less invasive and less expensive method capable of supplying sufficient material for analysis(4). In the case of microcalcifications, vacuum-assisted biopsy is less invasive than surgery and presents a lower smaller underestimation rate than simple core biopsy(1,5-7). A problem related to vacuum-assisted biopsy is its cost, not only in set-up and device acquisition but also the disposable consumables utilized the process. The cost of biopsies of lesions detected at screening programs may represent up to one-third of the program costs(8). The rational utilization of resources may produce savings and allow the application of such resources in other health-related activities, for example, extend the access to screening programs to more women(9). The tested system presents a considerably lower set-up cost, although the cost of disposable consumables is similar to that of other systems(10). No difficulty was observed with the cannula reinsertion for additional collections. Among the different vacuum-assisted devices currently available, some of them operate with a single cannula insertion, and others that operate with multiple insertions. In the first ones, there is a sampling chamber coupled to the cannula into where the collected fragment is moved, allowing its retrieval without removing the probe from the patients breast. In the presently tested device, the probe must be removed from the patient's breast for the retrieval of the collected sample, and then be reinserted for collection of a new fragment. This caused some preoccupation that the system might cause additional trauma for the breast because of the repeated probe insertions, and that the collection accuracy might be affected as a result of the target lesion displacement caused by the probe movements, but such complications were not observed. On average, each collection cycle took eight minutes to be completed, longer than the time spent with single cannula insertion systems. Part of this time was spent with the repeated probe insertions. This longer collection time may potentially limit the method accuracy due to patients' movements. The biopsy samples appropriateness suggests that accuracy was not compromised. It is important to remind that all biopsies performed in the present study were carried out on a dedicated examination table, on which the patient's immobilization tends to be more efficient. Hypothetically, when such procedure is performed with the patient on a sitting position, the probability of undesired movements is higher as the patient's torso retraction is not as well prevented by gravity force as it is with the dedicated prone examination table. In fact, the authors have observed that, during stereotactic biopsies with the patient on a sitting position (not included in this study), movements are not rare (which is easily noticed when the skin nick loses its alignment with the direction of the biopsy guide). The remaining question is whether, in the case of biopsies performed with the patient on a sitting position, the longer time required by the collection cycle with the portable device object of the present study, might interfere with the sampling accuracy. One limitation of the present study is that the low number of cases does not allow comparisons of rates of complications (among which the main one is the development of hematoma) with other vacuum-assisted biopsy methods. As the tested device is not equipped with resources for post-collection wash out and hematomas aspiration, hypothetically its use might result in a higher number of hematomas. A previous study has evaluated this matter and found that, with the handheld device, there was higher incidence of pain and lower incidence of early and late hematomas as compared with non-portable vacuum-assisted biopsy devices(11). Another limitation of the present study that is also related to the low number of cases is the fact that it did not compare the biopsies underestimation rate with that of non-portable devices. Finally, the tested portable vacuum-assisted biopsy device obtained appropriate samples in all cases, and is a lower-cost alternative for the performance of such biopsies. This was the first test of the device in our country, and the results are compatible with other studies in the literature that likewise have demonstrated its good performance(12,13).
REFERENCES 1. Burbank F, Parker SH, Fogarty TJ. Stereotactic breast biopsy: improved tissue harvesting with the Mammotome. Am Surg. 1996;62:738–44. [ ] 2. Liberman L, Gougoutas CA, Zakowski MF, et al. Calcifications highly suggestive of malignancy: comparison of breast biopsy methods. AJR Am J Roentgenol. 2001;177:165–72. [ ] 3. Liberman L, Smolkin JH, Dershaw DD, et al. Calcification retrieval at stereotactic, 11-gauge, directional, vacuum-assisted breast biopsy. Radiology. 1998;208:251–60. [ ] 4. Camargo Jr HSA. Diagnóstico por imagem da mama. Uma abordagem integrada. Rio de Janeiro: Revinter; 2008. [ ] 5. Fahrbach K, Sledge I, Cella C, et al. A comparison of the accuracy of two minimally invasive breast biopsy methods: a systematic literature review and meta-analysis. Arch Gynecol Obstet. 2006;274:63–73. [ ] 6. Hoorntje LE, Peeters PH, Mali WP, et al. Vacuum-assisted breast biopsy: a critical review. Eur J Cancer. 2003;39:1676–83. [ ] 7. Plantade R, Hammou JC, Fighiera M, et al. Underestimation of breast carcinoma with 11-gauge stereotactically guided directional vacuum-assisted biopsy. J Radiol. 2004;85(4 Pt 1):391–401. [ ] 8. Lindfors KK, Rosenquist CJ. The cost-effectiveness of mammographic screening strategies. JAMA. 1995;274:881–4. [ ] 9. Camargo Jr HSA, Camargo MMA. Reflexões sobre os custos dos programas de rastreamento do câncer de mama. Diagn tratamento. 2003;8:193–6. [ ] 10. Pistolese CA, Ciarrapico AM, Della Gatta F, et al. Cost-effectiveness analysis of two vacuum-assisted breast biopsy systems: Mammotome and Vacora. Radiol Med. 2009;114:743–56. [ ] 11. Salem C, Sakr R, Chopier J, et al. Pain and complications of directional vacuum-assisted stereotactic biopsy: comparison of the Mammotome and Vacora techniques. Eur J Radiol. 2009;72: 295–9. [ ] 12. Ghate SV, Rosen EL, Soo MS, et al. MRI-guided vacuum-assisted breast biopsy with a handheld portable biopsy system. AJR Am J Roentgenol. 2006;186:1733–6. [ ] 13. Hauth EA, Jaeger HJ, Lubnau J, et al. MR-guided vacuum-assisted breast biopsy with a handheld biopsy system: clinical experience and results in postinterventional MR mammography after 24 h. Eur Radiol. 2008;18:168–76. [ ] Received October 20, 2009. * Study developed at CDE Diagnóstico por Imagem, Campinas, SP, Brazil. |
|
Av. Paulista, 37 - 7° andar - Conj. 71 - CEP 01311-902 - São Paulo - SP - Brazil - Phone: (11) 3372-4544 - Fax: (11) 3372-4554