Radiologia Brasileira - Publicação Científica Oficial do Colégio Brasileiro de Radiologia
AMB - Associação Médica Brasileira CNA - Comissão Nacional de Acreditação
 Vol. 43 nº 2 - Mar. / Apr. of 2010
Vol. 43 nº 2 - Mar. / Apr. of 2010
|
ORIGINAL ARTICLE
|
|
Sonographic and hemodynamic findings of schistosomiasis mansoni: doppler sonography assessment in endemic areas |
|
|
Autho(rs): Leticia Martins Azeredo, Leonardo Campos de Queiroz, Carolina Coimbra Marinho, Maria Cristina Carvalho do Espírito Santo, Maria Cristina Chammas, Raiza Ruiz-Guevara, Aluizio Prata, Carlos Mauricio Figueiredo Antunes, José Roberto Lambertucci, Giovanni Guido Cerri |
|
|
Keywords: Schistosomiasis mansoni, Doppler sonography |
|
|
Abstract:
IPhD, Physician Assistant at Unit of Radiology and Imaging Diagnosis, Hospital das Clínicas da Universidade Federal de Minas Gerais (UFMG), Belo Horizonte, MG, Brazil
INTRODUCTION Schistosomiasis mansoni currently affects close to six million Brazilians. Approximately 5% to 10% of the infected patients develop the hepatosplenic form of the disease(1), which may progress with portal hypertension. Fibrosis, the scarring process that follows the inflammatory reaction caused by the presence of the parasites eggs on the walls of portal vessels, secondarily involving the bile ducts(2), is the anatomical substrate of this severe form of the disease. Differently from other chronic hepatopathies, the hepatic function is in general preserved in schistosomiasis, as no lesions occur in the hepatocyte. Upper gastrointestinal bleeding due to the rupture of esophagogastric varices resulting from hypertension is accountable for the morbimortality related to schistosomiasis mansoni. Therefore, vascular lesions determine the essential clinical aspects of this parasitosis. Ultrasonography (US), because of its capability to reveal the stage of the disease, has been widely utilized in studies of schistosomiasis mansoni morbidity in endemic regions(3–6). It is currently considered as the method of choice in populational studies(7), showing to be superior to physical examination for diagnosis, planning and monitoring of the disease management programs(8). Although the portal system vessels are clearly identified at US(7), hemodynamic data could only be obtained by means of invasive radiological techniques that are difficult to apply in populational studies(9,10). Doppler US is a milestone in the study of portal circulation, allowing noninvasive access to its morphological and functional aspects(11,12). Previous studies on portal hemodynamics in schistosomiasis mansoni by means of US-Doppler, all of them developed in hospital environment, focused on the evaluation of severe forms of the disease. Thus, it is important to develop more comprehensive investigation with Doppler velocimetry analysis covering the different forms of the disease, particularly the oligosymptomatic or asymptomatic ones, which are the majority of the cases in endemic regions. The present study, as the first one utilizing Doppler US in a field study and evaluating three areas with different endemicity levels, was aimed at identifying the sonographic and hemodynamic findings indicative of morbidity of schistosomiasis mansoni in the several stages of the disease.
MATERIALS AND METHODS Studied areas and sampling For this cross-sectional descriptive study, three endemic areas were selected in accordance with the endemicity criteria for schistosomiasis mansoni defined by the World Health Organization (WHO)(13), which takes into account the prevalence, parasite load and clinical presentation of the disease: 1. High endemicity – Brejo do Espírito Santo, rural area in the municipality of Santa Maria da Vitoria, in the State of Bahia (Brazil). 2. Medium endemicity – Chonim de Baixo, district of Governador Valadares municipality, in the State of Minas Gerais (Brazil). 3. Low endemicity – Municipality of Bananal, in the State of São Paulo (Brazil). The study sample included 554 patients with a history of infection by Schistosoma mansoni confirmed by stool examination for parasites. Exclusion criteria were: splenectomy, non-schistosomal chronic liver disease identified at US and presence of ascites. All participants or their parents/caregivers signed a term of free and informed consent. The project was approved by the Committee for Ethics in Research of Hospital das Clínicas da Faculdade de Medicina da Universidade de São Paulo. The sample included 325 male (58.7%) and 229 female (41.3%) volunteers aged between 10 and 92 years (mean age, 36.7 years) divided into three groups, according to the examination location: low endemicity area (n = 109), medium endemicity area (n = 255) e and high endemicity area (n = 190). Methods Conventional US – Longitudinal diameter of hepatic lobes spleen, diameter of the portal, splenic and superior mesenteric veins, gallbladder wall thickness, presence, grade, distribution and classification of periportal thickening were evaluated by two sonographers in compliance with the Niamey Working Group (2000) protocol(14,15), with a portable GE Logiq 100 unit (General Electric Medical Systems; Wisconsin, USA) with a convex 3.5 MHz transducer. Additionally, hepatic and splenic arteries diameters as well as the presence of collateral vessels were evaluated at mode B. Doppler US – Patency, direction and maximum flow velocity in portal (PVmax), splenic (SVmax), superior mesenteric veins (SMVmax) and collaterals, spectral pattern of flow in hepatic veins, peak systolic velocity and resistive index (RI) of hepatic and splenic arteries were evaluated by a single observer utilizing a portable GE Logiq-Book unit (General Electric Medical Systems; Wisconsin, USA) with a convex 3.5 MHz transducer. The spectral tracing of the hepatic veins was classified as triphasic (presence of reversal wave), biphasic (reduction in oscillatory amplitude with reversal wave loss) or monophasic (rectified flow)(16). Previous fasting and intestinal preparation was not required for patients examination because of the difficulty in standardizing such preparations in field studies. Statistical analysis The software SPSS 12.0 was used for data processing and analysis, considering 5% as significance level. In the statistical associations between categorical variables, the Pearson's chi-squared test was utilized for proportions comparison, while the Fisher's exact test was utilized for comparison of proportions with small frequencies. In the association of continuous variables, the non-parametric Mann-Whitney test was utilized for comparison between two groups, while the Kruskall-Walis test was utilized for comparison between three or more groups. The Spearman's correlation coefficient was utilized for comparison between two continuous variables. The non-parametric tests were chosen because of the asymmetric character of the tested variables.
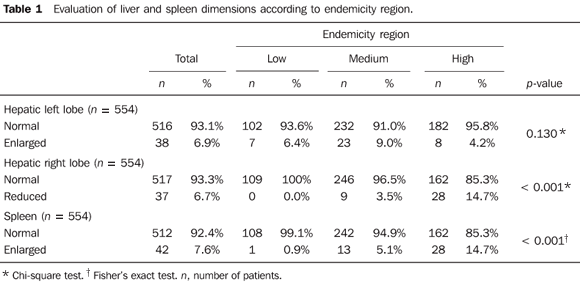
RESULTS Conventional US Mean longitudinal diameter of the right lobe was 126.9 mm, while that of the left lobe was 86.5 mm. The longitudinal diameter of the spleen ranged from 34 to 232 mm, with a mean value of 91.8 mm. Reduction of the right lobe (according to the Niamey Working Group, 2000 criteria)(14) and enlargement of the spleen (> 120 mm)(17) were most frequently observed in the highly endemic regions, with a statistically significant difference between the groups (p < 0.001) (Table 1). The prevalence of enlargement of the left lobe (> 110 mm)(17) was higher in the region of medium endemicity, without, however, statistically significant differences between the groups (p = 0.130) (Table 1).
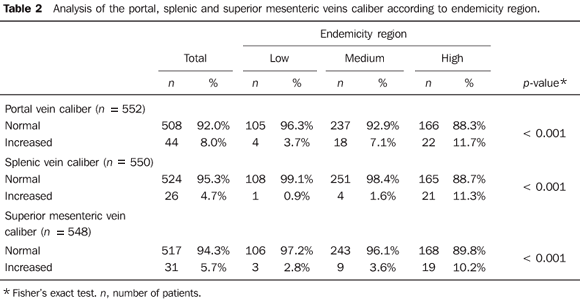
Mean diameter of the portal vein was 10.2 mm (ranging from 3.0 to 19.0 mm), while that of the splenic vein was 6.8 mm (3.0 mm to 21.0 mm) and that of the superior mesenteric vein was 7.4 mm (0.7 to 17.0 mm), with no statistically significant difference between regions in the cases of the portal vein (p = 0.523) and splenic vein (p = 0.322) and with statistically significant difference in the case of the superior mesenteric vein (p = 0.024). Dilatation of the portal vein (> 12 mm)(17), splenic vein and superior mesenteric vein (> 9 mm)(17) was more prevalent in the highly endemic region, with statistically significant differences between the groups (p < 0.001) (Table 2). It was not possible to measure the portal vein diameter in two patients, splenic vein diameter in four patients and superior mesenteric vein diameter in six patients.
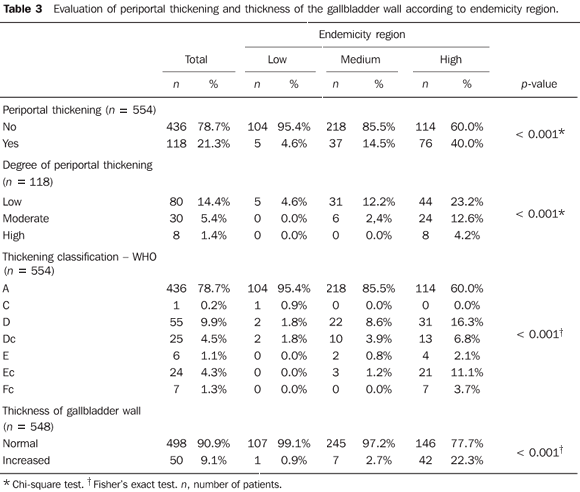
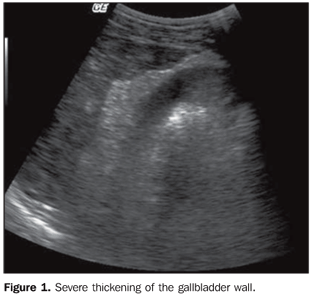
Mean hepatic artery diameter was 3.5 mm, ranging from 2.1 to 5.4 mm. Considering the reference value for normality (3.8 mm ± 0.8)(18), only 5 out of 481 patients (1.0%) presented increased hepatic artery diameter. The mean diameter of the splenic artery was 3.9 mm, with a minimum of 2.2 mm and a maximum of 12.3 mm. The gallbladder wall thickness ranged from 1 to 22 mm, with a mean value of 3.0 mm. In the stratification by endemicity region, a statistically significant difference was observed between the groups (p < 0.001), with thickening (> 5 mm)(19,20) being most frequently found in the highly endemic region (Table 3) (Figure 1). The gallbladder was evaluated in 548 patients, as 6 patients had been previously cholecystectomized.
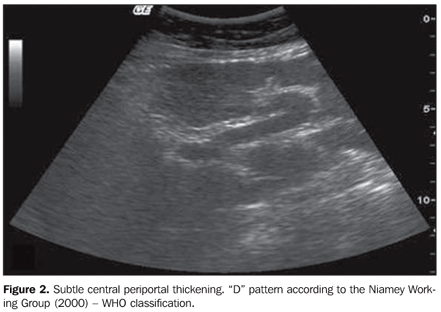
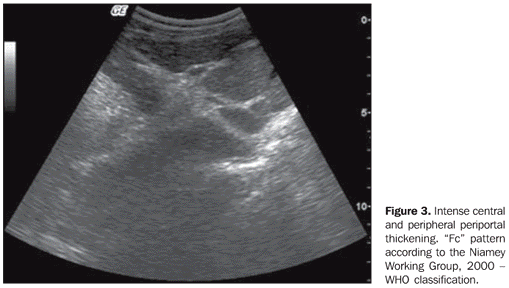
Results regarding the presence and degree of periportal thickening and its classification according to WHO involvement standards (Niamey Working Group, 2000)(14) demonstrated statistically significant difference between the groups (p < 0.001) (Table 3) (Figures 2 and 3). The thickening distribution was similar among the regions (p = 0.034).
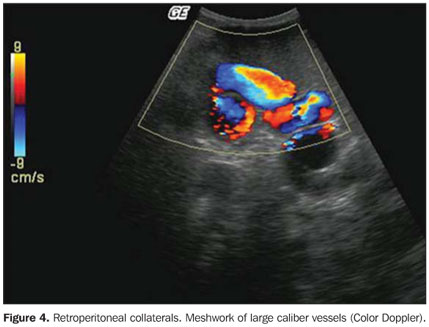
Doppler US Mean values for maximum flow velocity in portal system vessels were the following: PVmax = 23.1 cm/s (ranging from 12.0 to 55.6 cm/s), SVmax = 22.4 cm/s (11.9 to 44.5 cm/s) and SMVmax = 27.6 cm/s (14.1 to 55.2 cm/s). According to the reference values (>15 cm/s) for portal vein(21)flow velocity, 14/552 cases (2.5%) demonstrated decreased portal flow velocity, all of them in the highly endemic region. Portal thrombosis was identified in one patient from the high endemicity region, and low hepatofugal flow velocity was observed in another patient from the same region. The splenic vein and the superior mesenteric vein presented hepatopetal flow in all cases. In the arteries, the mean systolic peak velocity and RI observed were 80.1 cm/s and 0.70 in the hepatic artery, and 82.0 cm/s and 0.60 in the splenic artery, respectively. Considering the normal values of 0.69 ± 0.16(22), all the patients presented RI values within normality for the hepatic artery. Arteries were not identified in 84/554 patients (15.1%) in the case of the hepatic artery, and in 83/554 patients (15.0%) in the case of the splenic artery. In the highly endemic region, a significant association was observed between the presence and degree of periportal thickening and non identification of the hepatic artery (p < 0.001). The artery was identified in 106/161 (65.8%) of the patients without periportal thickening and in only 55/161 (34.2%) of those with the lesion. However, in the cases where the hepatic artery was identified and measured, there was no statistically significant difference in the vessel caliber (p = 0.223) between those that presented and those not presenting periportal fibrosis (mean diameter of 3.6 mm in both situations). The association between splenic artery diameter, RI and peak systolic velocity and the presence or not of splenomegaly, demonstrated statistically significant difference (p < 0.001) in the regions of medium and high endemicity. The mean diameter of the artery increased from 3.8 mm among the patients without splenomegaly, to 5.3 mm among those who presented such finding. Mean RI and mean peak systolic velocity were higher (0.70 e 101.4 cm/s) among the patients with splenomegaly, compared with those without this finding (0.60 e 80.5 cm/s). Considering the triphasic pattern as normal and the biphasic and monophasic patterns as altered(16), a spectral alteration was identified in the flow of the hepatic veins in 23.7% of the patients. The alteration show to be related to the presence and intensity of the periportal thickening, with higher frequency in the highly endemic region (30.8% versus 19.0% in the medium and 12.3% in the low endemicity regions), with statistically significant difference between the groups (p = 0.030). At Doppler, 15 collateral vessels were identified in 11 patients (4 with more than one type of collateral), all of them from the high endemicity region, representing 2.7% of the whole sample and 7.9% of the patients from that region. The most frequent collateral was the left gastric vein (7/15; 46%), followed by retroperitoneal (4/15; 27%) (Figure 4), short gastric (2/15; 13%), paraumbilical collaterals (1/15; 7%) and spontaneous intrahepatic portosystemic shunts (1/15; 7%) (Figure 5). All the gastric collaterals presented hepatofugal flow, with velocities higher than those of the portal vein.
DISCUSSION Based on sonographic findings, several investigations have attempted to establish the correlation between morbidity and endemicity in schistosomiasis mansoni, defining the incidence levels of the disease. The objective of the present study was establishing such correlation not only by means of the echographic alterations in the liver, spleen and vascular system, but also by the functional aspects of the portal circulation obtained by Doppler. In the present study, the frequency of sonographic signs of morbidity related to schistosomiasis mansoni demonstrated significant correlation with the endemicity level of the regions in practically all the evaluated parameters. Periportal thickening, splenomegaly, gallbladder wall thickening, right lobe reduction and portal vessels dilatation demonstrated prevalence strongly associated with the endemicity level of the regions and stage of the disease. All these markers presented higher frequency in the highly endemic region, with a significant difference between groups (p < 0.001) and with rates very similar to those reported in literature when compared to studies developed in regions with the same level of prevalence for schistosomiasis mansoni. Left lobe hypertrophy was the only variable at mode B that did not present significant difference between regions. Although attributed to the splenic hyperflow, and initially described in the hepatosplenic form(10), some authors had already reported the left lobe enlargement also in milder forms of the disease(23,24). The frequency of such alteration in the present study, although without statistical significance, was higher in the regions of medium and low endemicity, where most of the milder forms of disease are found. In these regions there was no significant correlation between left lobe enlargement and some variables indicative of splenic hyperflow, such as splenomegaly and dilatation of portal and splenic veins, indicating that this is not the cause for hypertrophy in these patients. Besides being the only parameter that did not show a difference between the regions, left lobe hypertrophy was also the only finding to present a lower prevalence in the highly endemic region. Correlating the presence of hypertrophy with the degree of periportal thickening, one could observe that among the patients with severe thickening, all of them from the highly endemic region, none presented left lobe enlargement. Such result indicates that, in advanced forms of the disease, the intense periportal fibrosis retracts the Glisson capsule, reducing the liver dimensions, which explains the lower frequency of left lobe hypertrophy in the highly endemic region. Therefore, it is possible to conclude that left lobe enlargement occurs in all stages of the disease, but with lower frequency in the more advanced cases, and that such an enlargement cannot be attributed exclusively to the splenic hyperflow(24). As regards the Doppler study, one emphasizes its viability and applicability in field studies. In spite of precarious conditions, the studies were performed with no setback. The technical problems observed were failure in the identification of some vessels, the non detection of flow in small caliber arteries and the impossibility of obtaining the appropriate angle for velocity measurement. In most cases such difficulties were related to obesity and lack of previous intestinal preparation. The visualization and flow mapping of almost the totality of veins was successful (portal vein, 99.6%; splenic vein, 99.3%; and superior mesenteric vein, 98.9%). With the arteries the success rate was lower (hepatic artery, 84.8%; and splenic artery, 85.0%), mainly because of the small caliber of these vessels or gas interposition. In the case of the hepatic artery, additional difficulties were observed in patients with fibrosis in the hepatic hilum. The mean value for maximum portal vein flow velocity in the present evaluation was 23.1 ± 6.3 cm/s. In only 14/552 (2.5%) patients, the maximum flow velocity was below the normality limit (15 cm/s)(21). Associating the diameter with the portal vein flow velocity, an inversely significant correlation was observed between the two variables (Spearman's coefficient: –0.262; p < 0.001), indicating that as the vein diameter increases, the flow velocity tends to decrease. Although flow volume has not been calculated in the present study, such results allow the conclusion, in agreement with Paranaguá-Vezozzo and Cerri(25), that the portal hyperflow described in the hepatosplenic form of the disease is related to the increase in diameter of the vessel, and not to the flow velocity. The spectral pattern of the flow in the hepatic veins is modulated by the cardiac cycle phases and is directly associated with hepatic parenchyma "elasticity". The alteration observed in the phasicity of the hepatic veins spectral curve in 23.7% of the patients in this study, was more frequent in the highly endemic region, with statistically significant difference between the groups (p = 0.030). The relation was significant as one associated the alteration of wave patterns to the presence and intensity of periportal thickening (p < 0.001). Such results demonstrate that periportal fibrosis present in schistosomiasis mansoni, in spite of not invading the hepatic lobes(26), reduces the "elasticity" of the parenchyma in the advanced forms of the disease, changing the spectral tracing of the hepatic veins. In the present investigation, collateral circulation was observed in 11 patients from the highly endemic region, corresponding to 5.9% of the patients from that group and 2% of the whole sample. Doppler US demonstrated higher accuracy than conventional US in the diagnosis of collateral circulation. Besides identifying a higher number of collaterals, a false-positive case of left gastric collateral, which, described at mode B, was not confirmed at Doppler. The recanalized paraumbilical vein, two cases of retroperitoneal collaterals and the portosystemic intrahepatic shunts were not identified by conventional US. An inverse, although not significant relation was observed between the collateral flow velocity and the portal vein flow velocity, confirming the relevant role played by the collateral in the reduction of the portal flow. Such relation was not observed only in the case of paraumbilical collateral which otherwise presented greater flow velocity in the portal vein and in its left branch. The participation of the hepatic artery in the schistosomiasis mansoni hemodynamics is controversial in the literature. Some authors report normal artery diameters(27), others report reduced diameters (10). Yet, a third group of authors suggest above-normal diameters(28). In the present study, the frequency of hepatic artery visualization was lower in the highly endemic region (84.7%), without, however, presenting significant difference between groups. In this region, the non identification of the artery was related to the presence and degree of periportal thickening (p < 0.001). However, once identified, the mean diameter and the dopplervelocimetric values of the artery were within the normality limits and did not present any difference between groups. Such results demonstrate that, in these patients, the limitation in the visualization of the artery was of a technical nature, caused by the increased echogenicity of the hepatic hilum due to fibrosis. Additionally, a significant and direct correlation was observed between maximum portal vein flow velocity and the peak systolic velocity of the hepatic artery (coefficient = 0.179) was found. This demonstrates the absence of an inverse relation between the portal and arterial flows in schistosomiasis, as in cirrhosis. Such results indicate that the hepatic artery, similarly to data reported by some authors(27,29), was within the normal limits in all the evaluated parameters and passively followed the hemodynamics of the schistosomal pathogenic process. With regards to the splenic artery, this is the first study to investigate its behavior by means of Doppler US at the different stages over the course of schistosomiasis mansoni. A significant correlation (p < 0.001) was observed between the presence of splenomegaly and increase in diameter, and systolic peak velocity and RI of this artery. If, by one side, the increase in diameter and systolic peak velocity indicate a greater volume of arterial flow (required to meet the demand of an enlarged spleen), on the other, the RI elevation reflects the difficulty of such volume to flow thru the splenic capillary bed. Such flow difficulty is directly related to the intrasplenic congestion caused by the increase in portal pressure. These results indicate that in hepatosplenic schistosomiasis as in cirrhosis(30), the splenic artery RI reflects the portal venous flow resistance, which may be an auxiliary parameter in the diagnosis of portal hypertension. In conclusion, the results obtained in the present study indicate that: Doppler US demonstrated to be viable and appropriate for noninvasive evaluation of hemodynamic alterations of schistosomiasis mansoni in field studies; sonographic signs of the disease morbidity are reliable parameters of the regions endemicity levels, except for the left lobe hypertrophy; the hepatic veins presented altered flow pattern in a significant number of patients, being such alteration related to the presence of intense periportal thickening; the hepatic artery did not present any alteration in the evaluated parameters, which suggests a passive behavior in the schistosomal pathogenic process; the splenic artery presented significant hemodynamic alterations secondary to portal hypertension. Finally, Doppler US has demonstrated to play a relevant role as an auxiliary tool in the study of morbidity related to schistosomiasis mansoni, contributing for a more precise description of the disease profile in endemic regions. Acknowledgments To Professor José Carlos Serufo (Faculdade de Medicina da Universidade Federal de Minas Gerais) for the invaluable contribution in the field research; to Dr. Sandra Costa Drummond (Secretary of Heath, State of Minas Gerais), Dr. Maria Laura Mariano de Matos, Dr. Mariana Benevides Santos Paiva and Dr. Ahraby Zaryff Morais Kansaon (Faculdade de Medicina da Universidade Federal de Minas Gerais), for the assistance in the development of the field work in Chonim de Baixo; to Dr. Izabela Voieta, Dr. Thais Sanai, Dr. Ana Carolina Figueiredo Pereira and Dr. Marina Nishi (Faculdade de Medicina da Universidade Federal de Minas Gerais), for their participation in the field work in Brejo do Espírito Santo, and to GE Healthcare, for the loan of the portable ultrasonography equipment.
REFERENCES 1. Coutinho AD. Hemodynamic studies of portal hypertension in schistosomiasis. Am J Med. 1968;44:547–56. [ ] 2. Sales DM, Santos JEM, Shigueoka DC, et al. Correlação interobservador das alterações morfológicas das vias biliares em pacientes com esquistossomose mansoni pela colangiorressonância magnética. Radiol Bras. 2009;42:277–82. [ ] 3. Lambertucci JR, Gespacher-Lara R, Pinto-Silva RAH, et al. The Queixadinha Project: morbidity and control of schistosomiasis in an endemic area in the northeast of Minas Gerais, Brazil. Rev Soc Bras Med Trop. 1996;29:127–35. [ ] 4. Gerspacher-Lara R, Pinto-Silva RA, Serufo JC, et al. Splenic palpation for the evaluation of morbidity due to schistosomiasis mansoni. Mem Inst Oswaldo Cruz. 1998;93 Suppl 1:245–8. [ ] 5. Scortegagna Junior E, Leão ARS, Santos JEM, et al. Avaliação da concordância entre ressonância magnética e ultra-sonografia na classificação da fibrose periportal em esquitossomóticos, segundo a classificação de Niamey. Radiol Bras. 2007;40: 303–8. [ ] 6. Gonzalez TD, Santos JEM, Sales DM, et al. Avaliação ultra-sonográfica de nódulos sideróticos esplênicos em pacientes esquistossomóticos com hipertensão portal. Radiol Bras. 2008;41:69–73. [ ] 7. Ricther J, Domingues ALC, Barata CH, et al. Report of the second satellite symposium on ultrasound in schistosomiasis. Mem Inst Oswaldo Cruz. 2001;96 Suppl:151–6. [ ] 8. Lambertucci JR, Cota GF, Pinto-Silva RA, et al. Hepatosplenic schistosomiasis in field-based studies: a combined clinical and sonographic definition. Mem Inst Oswaldo Cruz. 2001;96 Suppl:147–50. [ ] 9. Bogliolo L. A esplenoportografia na esquistossomíase mansônica hepatoesplênica, forma de Symmers. Rev Assoc Med Bras. 1957;3:263–9. [ ] 10. Mies S, Larsson E, Mori T, et al. Sistema porta e as artérias hepatica, esplênica e mesentérica superior na esquistossomose hepatoesplênica. Estudo angiográfico. Rev Hosp Clin Fac Med S Paulo. 1980;35:121–31. [ ] 11. Taylor KJ, Burns PN, Woodcock JP, et al. Blood flow in deep abdominal and pelvic vessels: ultrasonic pulsed-Doppler analysis. Radiology. 1985; 154:487–93. [ ] 12. Leão ARS, Santos JEM, Moulin DS, et al. Mensuração do volume de fluxo portal em pacientes esquistossomóticos: avaliação da reprodutibilidade do ultra-som Doppler. Radiol Bras. 2008;41: 305–8. [ ] 13. World Health Organization. The control of schistosomiasis. Technical Report Series, 728. Geneva: World Health Organization; 1985. [ ] 14. World Health Organization. Ultrasound in schistosomiais – a practical guide to the standardized use of ultrasonography for the assessment of schistosomiasis-related morbidity. Second International Workshop, October 22–26, 1996, Niamey, Niger. Geneva: World Health Organization, 2000. [ ] 15. Santos GT, Sales DM, Leão ARS, et al. Reprodutibilidade da classificação ultra-sonográfica de Niamey na avaliação da fibrose periportal na esquistossomose mansônica. Radiol Bras. 2007;40: 377–81. [ ] 16. Bolondi L, Li Bassi S, Gaiani S, et al. Liver cirrhosis: changes of Doppler waveform of hepatic veins. Radiology. 1991;178:513–6. [ ] 17. Cerri GG, Alves VAF, Magalhães A. Hepatosplenic schistosomiasis mansoni: ultrasound manifestations. Radiology. 1984;153:777–80. [ ] 18. Tziafalia C, Vlychov M, Tepetes K, et al. Echo-Doppler measurements of portal vein and hepatic artery in asymptomatic patients with hepatitis B virus and healthy adults. J Gastrointestin Liver Dis. 2006;15:343–6. [ ] 19. Machado M, Rosa ACF, Cerri GG. Doenças hepáticas difusas, hipertensão portal e transplante de fígado. In: Cerri GG, Oliveira IRS. Ultra-sonografia abdominal. São Paulo: Revinter; 2002. p. 56–124. [ ] 20. Pinto-Silva RA. A ultra-sonografia no diagnóstico da forma hepatesplênica da esquistossomose mansônica e de sua hipertensão portal [dissertação de mestrado]. Belo Horizonte: Universidade Federal de Minas Gerais; 1992. [ ] 21. Zironi G, Gaiani S, Fenyves D, et al. Value of measurement of mean portal flow velocity by Doppler flowmetry in the diagnosis of portal hypertension. J Hepatol. 1992;16:298–303. [ ] 22. Paulson EK, Kliewer MA, Frederick MG, et al. Hepatic artery: variability in measurement of resistive index and systolic acceleration time in healthy volunteers. Radiology. 1996;200:725–9. [ ] 23. Mackenjee MKR, Coovadia HM, Chutte CHJ. Clinical recognition of mild hepatic schistosomiasis in an endemic area. Trans R Soc Trop Med Hyg. 1984;78:13–5. [ ] 24. Paranaguá-Vezozzo DC. Avaliação hepática e hemodinâmica portal com Doppler duplex na esquistossomose mansônica [tese de doutorado]. São Paulo: Universidade de São Paulo; 1992. [ ] 25. Paranaguá-Vezozzo DC, Cerri GG. Duplex hemodynamic evaluation of hepatosplenic mansoni schistosomiasis. Mem Inst Oswaldo Cruz. 1992; 87:149–51. [ ] 26. Bogliolo L. Sobre o quadro anatômico do figado na forma hépato-esplênica da esquistossomose mansônica. Hospital (Rio de Janeiro). 1954;45: 283–306. [ ] 27. Bogliolo L. Terceira contribuição ao conhecimento do quadro anatômico do figado na esquistossomose mansônica hepato-esplênica. O comportamento da artéria hepática. Hospital (Rio de Janeiro). 1956;47:485. [ ] 28. Magalhães Fº A, Menezes H, Coelho RB. Patogênese da fibrose hepática na esquistossomose mansoni: estudo das alterações vasculares portais mediante modelo plástico. Rev Assoc Med Bras. 1960;6:284–94. [ ] 29. Coutinho A. Alterações hemodinâmicas na esquistossomose mansônica hepato-esplênica. J Bras Med. 1964;8:299–309. [ ] 30. Bolognesi M, Sacerdoti D, Merkel C, et al. Splenic Doppler impedance indices: influence of different portal hemodynamic conditions. Hepatology. 1996;23:1035–40. [ ] Received August 4, 2009. * Study developed in the Program of Post-Graduation in Radiology at Faculdade de Medicina da Universidade de São Paulo (HC-FMUSP), São Paulo, SP, Brazil. Field research involving three institutions: Faculdade de Medicina da Universidade de São Paulo (Department of Radiology and Tropical Medicine & Infectology), Faculdade de Medicina da Universidade Federal de Minas Gerais (Department of Medical Practice – Infectious and Parasitic Diseases), and Faculdade de Medicina do Triângulo Mineiro (Department of Medical Practice – Tropical Medicine). |
|
Av. Paulista, 37 - 7° andar - Conj. 71 - CEP 01311-902 - São Paulo - SP - Brazil - Phone: (11) 3372-4544 - Fax: (11) 3372-4554