Radiologia Brasileira - Publicação Científica Oficial do Colégio Brasileiro de Radiologia
AMB - Associação Médica Brasileira CNA - Comissão Nacional de Acreditação
 Vol. 42 nº 2 - Mar. / Apr. of 2009
Vol. 42 nº 2 - Mar. / Apr. of 2009
|
ORIGINAL ARTICLE
|
|
Rectal dose assessment in patients submitted to high-dose-rate brachytherapy for uterine cervix cancer |
|
|
Autho(rs): Jetro Pereira de Oliveira, Luiz Antonio Ribeiro da Rosa, Delano Valdivino Santos Batista, Lúcia Helena Bardella, Arnaldo Rangel Carvalho |
|
|
Keywords: Rectum, Brachytherapy, HDR, Thermoluminescent dosimetry, Uterine cervix cancer |
|
|
Abstract:
IMaster, Fellow PhD degree, Faculdade de Medicina da Universidade Federal do Rio de Janeiro (UFRJ), Rio de Janeiro, RJ, Brazil
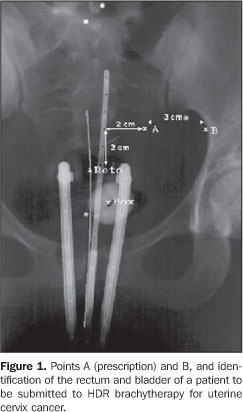
INTRODUCTION The uterine cervix is a cylindrical and narrow portion of the uterus, measuring 2-4 cm in length, connecting to the anterior wall of the vagina, in most of cases lying perpendicularly to this structure. It joins the lower segment of the uterus at the isthmus level, where a subtle narrowing of the lumen is observed. Uterine cervix cancer represents a continuing challenge to the clinical practice. According to the Instituto Nacional de Câncer (INCA), in 2008 19,000 new cases of the disease are expected to be detected in Brazil(1). Among Brazilian women, uterine cervix cancer is the third most frequent type of cancer. Not considering non-melanoma skin tumors, uterine cervix cancer is the most common one in the Northern region of the country. In the Southern, Western and Northeastern regions this form of cancer is the second most common and in the Southeastern region it is the fourth most common(2). With approximately 500,000 new cases per year in the world, uterine cervix cancer is the second most frequent form of cancer affecting women, causing about 230,000 deaths per year. The incidence of this disease in less developed countries is twice as high as that in more developed countries(3). One of the methods used in the treatment of uterine cervix cancer with ionizing radiation is radiotherapy. Radiotherapy is divided into teletherapy and brachytherapy. In brachytherapy, the sources placed next to the tumor are radioisotopes whose radiation penetrates the tissues of interest, releasing energy locally and leading to the death of neoplastic(4) cells. Brachytherapy is typically utilized as a complementary treatment after the patient has been submitted to teletherapy. In January of 2001, the Brazilian radiotherapy completed ten years of experience with high-dose-rate (HDR) brachytherapy, defined as the treatment whose doses are higher than 0.2 Gy/min(5). Undoubtedly, radiotherapy is an effective option for the treatment of uterine cervix cancer, but also it poses a risk to radiosensitive organs adjacent to the uterus, such as the rectum and the bladder. In the case of the rectum, a high dose can cause complications, with possibilities ranging from episodic diarrhea, rectal spasmus and occasional bleeding, up to local ulceration, partial stenosis, reoccurring abundant hemorrhage, with necrosis and obstruction and, finally, development of rectovaginal fistula. An appropriate treatment plan can assure with a high degree of accuracy, a high radiation dose to the tumor, with simultaneous protection against high dose exposure for the rectum and bladder. The planning systems utilized in brachytherapy for treatment of uterine cervix cancers are based on mathematical models which are naturally limited. Whenever possible, procedures for evaluation of doses to organs at risk are recommended so that it becomes possible to compare these dose values with those established in the treatment plan. Thus, not only the plan correctness is evaluated, but also the irradiation procedure utilized in the patient is validated. This can be done by adopting in vivo procedures that is, assessing the doses during treatment by means of thermoluminescent dosimeters (TLDs), radiophotoluminescent glass dosimeters (RPLGDs), metal oxide semiconductor field effect transistors (MOSFETs) or diodes(6-9). In the present study, the authors proposed the utilization of TLD LiF:Mg,Ti,Na detector in the form of a powder, for in vivo measurement of doses to the rectum of patients during the treatment for uterine cervix cancer utilizing a 192Ir source in HDR brachytherapy. Criterion for limitation of doses to the rectum In brachytherapy, because of the non-homogeneous dose distribution, the dose prescription is more complex than in teletherapy. Different systems have been developed with different prescription items. The Report 38 of International Commission of Radiation Units and Measurement (ICRU)(10) suggests the standardization of the manner in which dose and treatment dose are reported. Such report includes a number of items that should be included in the prescription of intracavitary therapy. The technique description, including dose and fractioning, and the temporal ratio with the implant, the type of intracavitary system applied, the isotope utilized, the absorbed dose in a number of relevant points to the tumor management, the dose to the rectum and to the bladder are some of the items that should be included in the report of intracavitary therapy. The points of relevance in the management of the tumor are located in the pelvic wall and in the lymphatic Fletcher trapezoid. The critical reference points for the bladder and the rectum are defined in the vesical trigone (on the surface of the Foley catheter balloon filled with 7 ml of contrast agent) and on the anterior rectal wall (0.5 cm beyond the posterior vaginal wall, on a line passing by the medium point of the colpostat source). The vesical trigone is a geometric figure formed by an inverted triangle, whose vertices correspond to the points where the urethers connect to the bladder and to the urethral orifice in the bladder(10). One of the first essential steps to determine the dose distribution in brachytherapy is based on the determination of the source positioning in relation to the target volume and other anatomic characteristics of interest. The point A prescription, in spite of being criticized by many authors and by the ICRU Report 38(10), is still the most frequently utilized method of dose prescription. Point A was originally described as being located 2.0 cm above the vaginal fornix and 2.0 cm laterally in relation to the uterine canal, having been defined to reflect an average dose inside the paracervical triangle containing critical vascular structures (tolerance pyramid). Ideally, point A represents the intersection of the urether with the uterine artery. The point B, 2.0 cm above and 5.0 cm laterally, following the same origin system utilized in the localization of point A, represents the pelvic wall and, particularly, the lymph nodes, and can be seen on Figure 1. The dose to point B typically corresponds to one third of the dose applied to point A.
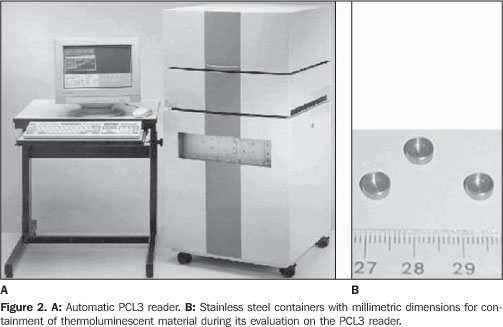
MATERIALS AND METHODS The thermoluminescent powder utilized was lithium fluoride doped with magnesium, titanium and sodium (LiF:Mg,Ti,Na). This compound is produced by the French company Philitech (Philitech; Paris, France) under the code DTL937. This product is enriched with 7Li (99.994%). The pre-irradiation thermal therapy utilized for material regeneration was 450 °C for three hours. An ETT oven (Fimel; Paris, France) was utilized for this thermal therapy. The thermoluminescent powder, once irradiated, is evaluated in a PCL3 automatic reader. The post-irradiation thermal therapy is carried out in the thermoluminescent reader itself at a temperature of 125 °C for five seconds. This temperature is lower than the temperature utilized in the evaluation of the TLDs which is 440 °C. The equipment is produced by the French company Fimel, and can read about 34 mg of the material placed in small stainless steel containers, as seen on Figure 2.
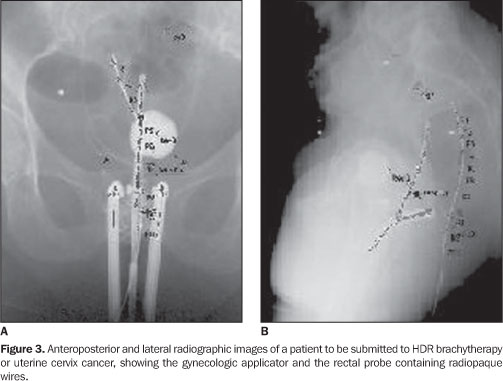
The dose limitation criterion adopted by INCA was four applications at one-week intervals, each one of 700 cGy (prescribed dose on point A), with a maximum of 65% of this dose reaching the rectum. For the bladder and the sigmoid colon, the dose is limited to 70% and 55% respectively, to the dose on point A. Before assessing the doses in the rectum, the reproducibility and linearity of the dosimetric system was evaluated. The experiment to evaluate the linearity was conducted in a cubic acrylic phantom measuring 38.0 × 38.0 × 30.5 cm3, filled with water. Approximately 34 mg of the dosimetric powder were placed in 20.0 cm long capillaries with 3.0 mm in diameter. The powder was placed in the posterior part of the capillaries. These were positioned at 3.0 cm from the 192Ir source and were irradiated three times at different dose levels, from 4.9 to 902.3 cGy. Blocks of "solid water" were utilized for TLDs calibration as a function of the absorbed in water. This procedure was chosen in order to minimize the uncertainty level in the dosimeter positions relative to the source, considering that because of the high dose gradient, the accuracy in such positioning is very important. The use of solid water in the current study, instead of normal liquid water, was based on the results of Meli et al.(11), who, by using Monte Carlo simulation, demonstrated the appropriateness of the simulator material for measurements with 192Ir sources. The thermoluminescent powder samples were calibrated at a 6.0 cm-distance from the 192Ir source. The absorbed dose values in water in the points where the samples of thermoluminescent material were placed, was calculated using the mathematical formulas developed in the Report 51 of the American Association of Physicists in Medicine (AAPM)(12), based on measurement of air kerma rates at 10.0 cm from the source using the Farmer chamber with the calibration factor determined by Maréchal(13-15). After TLDs calibration for the energy spectrum of gamma radiation from 192Ir, the method was tried with patients undergoing treatment at INCA. In the routine gynecological procedure, before the treatment is actually initiated a probe is introduced into the patient's rectum. This probe is a disposable tube made of non-toxic siliconized polyvinyl chloride and sterilized by gamma radiation. Radiopaque wires are placed within this probe in the gynecologic applicators. Before exposure to therapeutic 192Ir radiation, the patient is exposed to x-rays, and anterior-posterior and lateral images are obtained for therapy planning, as seen on Figure 3. Therefore, the doses delivered along the rectum, more precisely along the radiopaque wire within the rectal probe, can be planned. By replacing the wire by the capillary containing thermoluminescent powder, before the patient is exposed to 192Ir, it is possible to evaluate the dose along this organ during treatment and comparing the experimental values with those determined by the planning system, allowing a new planning for the following therapy application, in cases where the measured doses were higher than the limit value for the rectum. The capillary utilized for measuring the rectal dose is 20.0 cm long and 3.0 mm in diameter. It is divided in ten small compartments, each filled with 34 mg of the dosimetric powder. The capillary is very carefully introduced into the rectal probe, so that such probe is kept in the same position it held when the radiopaque wire was in place. Figure 4 shows a drawing of this capillary.
Before collaborate with the investigation, all patients were explained about the type of experiment to be developed, and agreed in participating. In total, six patients participated in the investigation.
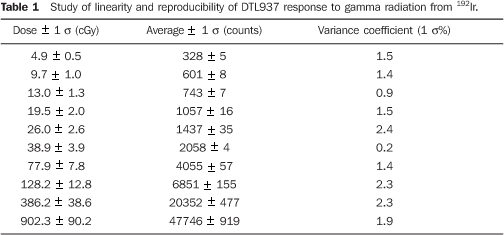
RESULTS Table 1 presents the study results on the reproducibility of the DTL937 response to gamma radiation energy from 192Ir. Three irradiations were made for each value of absorbed dose. The thermoluminescent response of the dosimetric material presents good reproducibility, lower than 2.4% for the considered dose interval, between 4.9 and 902.3 cGy (mean and standard deviation: 1.6 ± 0.7%).
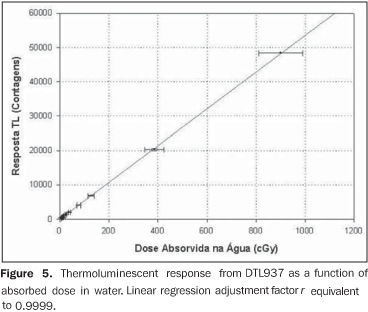
The TLD response presents a linear behavior, making it unnecessary to apply linearity correction factors to the results as it can be seen on Figure 5. The linear regression adjustment factor (r) is 0.9999.
Table 2 presents dosimetry results for the rectum obtained from patients submitted to HDR brachytherapy for uterine cervix cancer. The limit dose determined for the rectum according to the criterion adopted by INCA is shown, as well as the maximum dose obtained by the planning system for the rectum, the maximum dose measured in the rectum with TLDs, the average dose obtained by means of the planning system for the rectum, and the average dose in the rectum measured by the TLDs. Although the TLDs response presents a good reproducibility, (1.6 ± 0.7)%, on average, the uncertainty in experimental measurements and in the dose values obtained by the planning system achieves about 10%. The TLDs calibration was based on mathematical formulas developed in the AAPM Report 51(12). The planning system utilized for the calculation of delivered dose to the target volume and to the rectum in brachytherapy is based the same formulas. In the AAPM(12) document, with basis on the quadratic propagation of uncertainty involved in the formulas, estimated uncertainty level is 10% for the calculated dose.
DISCUSSION Considering the uncertainty associated with measured doses and the planned ones (Table 2), one observes that in the six cases included in the present study the doses delivered to the patients rectums were at a maximum, equal to the limit doses determined for the organ, following the adopted criterion and taking the estimated uncertainties into account. As far as the average dose in the rectum is concerned, the measurements presented a good agreement with the planned values. The same can be observed in relation to maximum dose values. In the cases of patients 1 and 2, there are bigger differences between planned values and the measured ones. Possibly, because internal movements of the organs, the positioning of the thermoluminescent dosimeters may not have been as accurate as in the other patients. It is extremely important that the dosimeters are positioned in the same point utilized by the planning system for dose calculation. The uncertainty of experimental measurements and of dose values obtained by the planning system achieves about 10%. This is due to the inherent uncertainty of dose calculation based on the AAPM document TG 43(12) which is approximately 10%. Lambert et al.(7) have studied different dosimeters for in vivo dosimetry applications during treatment with HDR brachytherapy. Thermoluminescent, MOSFETs, diamonds and scintillation detectors were evaluated. The diamond detectors presented the most accurate results; however they are too large for dose measurements in HDR brachytherapy, in which the dose gradients are very high. The MOSFETs presented errors rates between 30% and 40% in measurements at more than 5.0 cm from the source, although for measurements at a distance between 2.0 and 5.0 cm, the errors rate were at the order of 5%. The scintillation detector presented a dosimetric accuracy of 3% for measurements made between 1.0 and 10.0 cm from the brachytherapy source. The authors' criticism in relation to TLDs was due to their incapacity of carrying out real-time measurements, although their dosimetric performance was better than the MOSFETs performance. It is important note that diamond and scintillation detectors are not commonly utilized in radiotherapy centers, and that TLDs, diodes and MOSFETs are most widely utilized in medical physics for in vivo measurements(16). Sakata et al.(6) have measured doses in the rectum utilizing semiconductor dosimeters during intracavitary brachytherapy procedures for treatment of gynecologic cancer in 105 patients. The results indicate differences of up to 5% between planned and measured doses for 30.8% of the patients. This difference may reach 10% for 56% of the patients and up to 20% for 85% of the patients. For 15% of the patients, the differences between planned and measured doses were higher than 20%. Waldhäusl et al.(9), using diodes, have measured the dose in the rectum in 55 HDR brachytherapy procedures in patients with gynecologic cancer. Differences from -31% to +90% were found between measured values and calculated values for delivered doses to the organ during treatment for uterine cancer. The main reason for such high differences was due to uncertainty in the detectors positioning. Contrary to results reported by Waldhäusl et al.(9) and Sakata et al.(6), in the present study the differences between planned dose values and measured dose values were always bellow 8%, with several values lower than 3.5%. Forty four percent of the differences between calculated and measured dose values in the rectum by Sakata et al.(6) were greater than 10%, and 15% of these were greater than 20%. Waldhäusl et al.(9) have found differences ranging from -31% to +90% between calculated and measured doses in the rectum of patients submitted to HDR brachytherapy. It is important to note that Sakata et al.(6) have studied 105 patients, and the results reported by Waldhäusl et al.(9) were based on 55 applications of HDR brachytherapy. In the present study, only six patients were studied and, therefore, its statistical significance is very poor when compared to that of the mentioned bibliography(6,9). Thus, based on the present results one cannot say that the procedure herein described is superior to the procedures utilized by the mentioned authors(6,9), however, the method has shown to be appropriate for the evaluation of rectal doses, and can indicate a need for reevaluation of the therapy plan in the case of detection of rectal doses considerably higher (> 10%) than planned. Sakata et al.(6) and Waldhäusl et al.(9) have utilized diodes in their studies. It is important to mention that according to Lambert et al.(7), the diodes are less accurate than TLDs in in vivo dose evaluations. In the present study, thermoluminescent dosimetry was the technique utilized. TLDs have the inconvenience of not allowing a real-time dose evaluation, contrary to diodes or MOSFETs. However, due to the fact that they do not require wires and electrometers during measurement, they are more suitable to in vivo measurements, causing less trouble to patients. They are also smaller than the diodes, which is also an advantage for a dosimeter for in vivo dose evaluations. The MOSFETs can also be very small, such as the micro-MOSFETs. TLDs are also less subject to patient temperature variations when compared to diodes(17). The dosimetric procedure described in the present study allows the evaluation of the dose delivered to the rectum as a consequence of the HDR brachytherapy for uterine cervix cancer. Such evaluation makes it possible for the medical team to make decisions on the best approaches for the continuation of the treatment administered to the patients, when the measured doses are higher than the planned ones, in order to protect the organ, which contributes to minimizing complications that may occur when the rectum is exposed to high radiation doses.
CONCLUSION The thermoluminescent dosimetry system utilized in the present study, is simple and easy to use when compared to other possible dosimetric techniques applicable to in vivo dosimetry in the rectum, and has shown to be efficient in the evaluation of rectal dose in patients submitted to HDR brachytherapy for uterine cervix cancer, allowing the reevaluation of the therapy plan between the first and second weekly applications, when doses above the limit (> 10%) are measured in the organ to be protected. Acknowledgements To Comissão Nacional de Energia Nuclear (CNEN) for the financial support, to Dr. Andrés Reinaldo Rodrigues Papa, first Coordinator of the Comissão de Pós-Graduação do Instituto de Radioproteção e Dosimetria (CPG/IRD), for the support given to the first author during his Master's course, to the brachytherapy team at INCA, for the constant support during the measurements phase, as well as to the persons who extended their help in the conception of this study.
REFERENCES 1. Instituto Nacional de Câncer. Estimativa 2008: incidência de câncer no Brasil. Rio de Janeiro: INCA; 2007. p. 24. [ ] 2. Instituto Nacional de Câncer. Estimativa 2008: incidência de câncer no Brasil. Rio de Janeiro: INCA; 2007. p. 32. [ ] 3. Salvajoli JV, Souhami L, Faria LS. Radioterapia em oncologia. 1ª ed. Rio de Janeiro: Editora Médica e Científica; 1999. [ ] 4. Calcina CSG, Almeida A, Rocha JRO. Análises de protocolos de braquiterapia, por alta taxa de dose, do controle de qualidade de alguns serviços locais, baseados no TG40, TG56 e ARCAL XXX. Radiol Bras. 2001;34:225-32. [ ] 5. Esteves SCB, Oliveira ACZ, Feijó LFA. Braquiterapia de alta taxa de dose no Brasil. Radiol Bras. 2004;37:337-41. [ ] 6. Sakata K, Nagakura H, Oouchi A, et al. High-dose-rate intracavitary brachytherapy: results of analyses of late rectal complications. Int J Radiat Oncol Biol Phys. 2002;54:1369-76. [ ] 7. Lambert J, Nakano T, Law S, et al. In vivo dosimeters for HDR brachytherapy: a comparison of a diamond detector, MOSFET, TLD, and scintillation detector. Med Phys. 2007;34:1759-65. [ ] 8. Nose T, Koizumi M, Yoshida K, et al. In vivo dosimetry of high-dose-rate interstitial brachyther-apy in the pelvic region: use of radiophotolumi-nescence glass dosimeter for measurement of 1004 points in 66 patients with pelvic malignancy. Int J Radiat Oncol Biol Phys. 2008;70: 626-33. [ ] 9. Waldhäusl C, Wambersie A, Pötter R, et al. In-vivo dosimetry for gynaecological brachytherapy: physical and clinical considerations. Radiother Oncol. 2005;77:310-7. [ ] 10. International Commission on Radiation Units and Measurements. Dose and volume specification for reporting intracavitary therapy in gynaecology. ICRU Report 38. Bethesda: ICRU; 1985. p. 1-16. [ ] 11. Meli J, Meigooni A, Nath R. On the choice of phantom material for the dosimetry of 192Ir sources. Int J Radiat Oncol Biol Phys. 1988;14: 587-94. [ ] 12. American Association of Physicists in Medicine. Dosimetry of interstitial brachytherapy sources: recommendations of the AAPM Radiation Therapy Committee Task Group No. 43. AAPM Report No. 51. Woodbury: American Institute of Physics; 1995. [Reprinted from Med Phys. 1995;22:209-34] [ ]. 13. Maréchal MH. Recomendações para calibração de fontes de 192Ir de alta taxa de dose [tese de doutorado]. Rio de Janeiro: Universidade do Estado do Rio de Janeiro; 2000. [ ] 14. Ferreira IH, Almeida CE, Marre D, et al. Monte Carlo calculations of the ionization chamber wall correction factors for 192Ir and 60Co gamma rays and 250 kV x-rays for use in calibration of 192Ir HDR brachytherapy sources. Phys Med Biol. 1999;44:1897-904. [ ] 15. Maréchal MH, Almeida CE, Ferreira IH, et al. Experimental derivation of wall correction factors for ionization chambers used in high dose rate 192Ir source calibration. Med Phys. 2002;29:1-5. [ ] 16. Kron T. Applications of thermoluminescence dosimetry in medicine. Radiat Prot Dosimetry. 1999;85:333-40. [ ] 17. Mayles WPM, Heisig S, Mayles HMO. Treatment verification and in vivo dosimetry. In: Williams JR, Thwaites DI, editors. Radiotherapy physics in practice. 2nd ed. New York: Oxford University Press, Inc.; 2000. p. 220-46. [ ] Received September 14, 2007. * Study developed at Instituto Nacional de Câncer (INCA), Rio de Janeiro, RJ, Brazil. |
|
Av. Paulista, 37 - 7° andar - Conj. 71 - CEP 01311-902 - São Paulo - SP - Brazil - Phone: (11) 3372-4544 - Fax: (11) 3372-4554