Radiologia Brasileira - Publicação Científica Oficial do Colégio Brasileiro de Radiologia
AMB - Associação Médica Brasileira CNA - Comissão Nacional de Acreditação
 Vol. 55 nº 3 - May / June of 2022
Vol. 55 nº 3 - May / June of 2022
|
ORIGINAL ARTICLE
|
|
Assessment of the performance of the O-RADS MRI score for the evaluation of adnexal masses, with technical notes |
|
|
Autho(rs): Patrick Nunes Pereira1,a; Adriana Yoshida1,b; Luís Otavio Sarian1,c; Ricardo Hoelz de Oliveira Barros2,d; Rodrigo Menezes Jales1,e; Sophie Derchain1,f |
|
|
Keywords: Magnetic resonance imaging; Adnexal diseases/diagnostic imaging; Ovarian neoplasms/diagnostic imaging. |
|
|
Abstract: INTRODUCTION
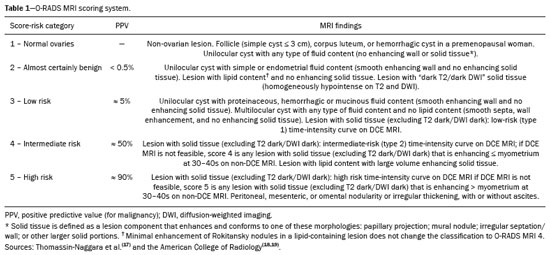
Adnexal masses are common findings in clinical practice(1,2). The vast majority of adnexal masses are benign, malignant masses accounting for only a small proportion(3,4). In 2020, the estimated number of cases of ovarian cancer worldwide was only 313,000(5). Excluding malignancy of an adnexal mass through preoperative examinations is crucial for proper screening and treatment planning. It is recommended that a woman with a suspicious adnexal mass be referred to a surgeon specializing in gynecologic oncology(6). In most cases, screening for the risk of malignancy of an adnexal mass can be performed effectively by transvaginal ultrasound and the use of targeted algorithms, especially if the simple rules established by the International Ovarian Tumor Analysis (IOTA) group are applied(7,8). However, approximately 20% of adnexal masses are considered indeterminate on ultrasound(9,10). Adnexal masses that are considered indeterminate represent a dilemma for the entire team that treats the affected patient, because such patients are at risk of unnecessary surgery. However, if the watchful waiting approach is adopted, the “window” of opportunity to diagnose cancer at an early stage may be missed. There is also a risk that the patient will be subjected to a surgical procedure performed by a non-specialist, if there is a false-negative test result or a lesion of non-ovarian origin(6,11). According to the European Society of Urogenital Radiology(12,13), magnetic resonance imaging (MRI) of the pelvis is indicated to assess an adnexal tumor that is considered indeterminate on transvaginal ultrasound. In 2013, Thomassin-Naggara et al.(14) presented a diagnostic algorithm for adnexal lesions that combines morphological features and functional MRI aspects to assign a numerical score. The system was initially called the ADNEX MR SCORING system and had five distinct categories (corresponding to scores from 1 to 5): categories 1, 2, and 3 were related to (probably) benign masses; and categories 4 and 5 were related to adnexal masses considered indeterminate or suspicious for malignancy. At cutoff scores of 4 and 5, the score had excellent accuracy (with a sensitivity of 93.5% and a specificity of 96.6%) for the detection of malignancy in an adnexal mass(14). However, some technical aspects, such as the time-intensity of dynamic contrast-enhanced (DCE) studies of contrast-enhanced sequences (perfusion studies), which constituted one of the central elements of the ADNEX MR SCORING system, likely would have limited its use on a larger scale(15). Studies have shown that it is possible to use less complex contrast-enhanced sequences, with no loss of accuracy(16). Recently, the ADNEX MR SCORING system was validated in a large prospective multicenter study conducted by the original authors(17); some MRI aspects and parameters were improved, after which the score was adopted by the American College of Radiology, at which point it was renamed the Ovarian-Adnexal Reporting and Data System MRI (O-RADS MRI) score(18,19). Some modifications were made; the new score incorporated the possibility of using lower temporal resolution in the DCE studies and even of performing a visual analysis of the enhancement pattern when a DCE study is not feasible(18). The O-RADS MRI score was designed to simplify and standardize the reporting of MRI of adnexal masses, in order to provide the clinician with the information necessary for the most appropriate management of patients, similar to the Breast Imaging Reporting and Data System, the result of which is linked to the clinical management of breast lesions(20). In the O-RADS MRI score(18), the absence of a suspicious adnexal lesion receives a score of 1; an adnexal lesion that is almost certainly benign receives a score of 2; a low-risk lesion receives a score of 3; an intermediate-risk lesion receives a score of 4; and a high-risk lesion receives a score of 5. In their subsequent study, Thomassin-Naggara et al.(17) found that, for the detection of malignancy, a score of 4 or 5 had a sensitivity of 93.0% (95% CI: 89–96) and a specificity of 91.0% (95% CI: 89–93). In this article, we evaluate the accuracy of the O-RADS MRI for evaluating adnexal masses in a large sample of lesions. We also provide technical notes on the updates in relation to the original score(14). MATERIALS AND METHODS This was a prospective study conducted at the Centro de Atenção Integral à Saúde da Mulher/Hospital da Mulher Prof. Dr. J. A. Pinotti, a tertiary cancer center operated by the Faculdade de Ciências Médicas da Universidade Estadual de Campinas (Unicamp), in the city of Campinas, SP, Brazil. The study was approved by the Unicamp Research Ethics Committee (Reference nos. 1092/2009 and 008/2010). All participating patients gave written informed consent. We randomly recruited women who were referred to our hospital for investigation of an adnexal mass between February 2014 and December 2020. To avoid any selection biases, we ensured that the recruiter had no knowledge of the clinical data (e.g., time of evolution), laboratory test results (serum levels of CA-125), or findings on imaging (pelvic ultrasound) for any given patient. An ultrasound evaluation of the pelvis was scheduled for each of the women enrolled. After ultrasound, 257 cases were scheduled for MRI, which was performed at the Hospital Estadual Sumaré, in the city of Sumaré, SP, Brazil, a Unicamp-affiliated hospital located near the Hospital da Mulher Prof. Dr. J. A. Pinotti. When indicated, diagnostic or therapeutic surgical procedures were performed. The indication for surgery was based on the results of the clinical examination; preoperative biomarker levels; the ultrasound findings, evaluated with the IOTA simple rules, as described by Timmerman et al.(7); and the MRI results (practitioners did not have access to MRI scoring results). Figure 1 shows the patient selection process. Of the 257 women initially enrolled, 14 were lost to follow-up. Therefore, data for 243 women, with a total of 287 adnexal masses, were included in the study. Of the 169 patients (with 190 adnexal masses) for whom a histological diagnosis was made, 62 were found to have a single malignant adnexal tumor, 14 were found to have bilateral malignant adnexal tumors, 86 were found to have a single benign adnexal tumor, and 7 were found to have bilateral benign adnexal tumors. A team of pathologists specializing in pelvic neoplasms made the final histological diagnosis, in accordance with the guidelines established by the WHO Classification of Tumours of Female Reproductive Organs(21). Of the 74 patients (with 97 adnexal masses) who did not undergo an invasive procedure and were followed to evaluate their clinical evolution, one had four adnexal masses, two had three adnexal masses, 16 had two adnexal masses, and 55 had a single adnexal mass. The last follow-up evaluation was in December 2020. 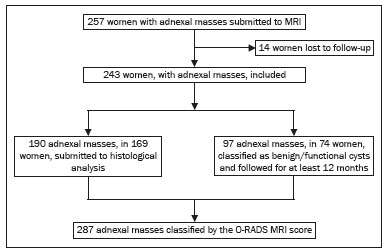 Figure 1. Flow chart of the patient selection process. The MRI scans, surgical procedures, and histopathological analyses were performed at the Hospital da Mulher Prof. Dr. J. A. Pinotti. More than one tumor was found in 40 women, and the O-RADS MRI score was calculated for each mass separately, as suggested by its authors(17). All MRI assessments were performed before the histological diagnosis was known or the decision for clinical follow-up had been made. The reference standard was the histopathological diagnosis in the adnexal masses submitted to surgery (n = 178) and in those submitted to percutaneous biopsy (n = 12). For the adnexal masses not subjected to histopathological examination (n = 97), the criteria for benign disease were based on clinical and imaging data obtained over a period of at least 12 months, following the usual clinical care protocols of the institution. MRI All MRI scans were acquired in a 1.5-T scanner (Signa HDxt; GE Healthcare, Milwaukee, WI, USA) with a pelvic phased-array coil. Prior the MRI scans, patients fasted for 3 h. No antispasmodic agents were used; nor was vaginal or rectal contrast administered. We used a protocol aimed at assessing adnexal masses, which consisted of T2-weighted multiplanar (axial, sagittal, and coronal) sequences, in-phase and out-of-phase T1-weighted sequences, diffusion-weighted sequences (b = 0, 500, and 1,000 s/mm2), and T1-weighted sequences, with and without fat saturation, before and after intravenous contrast administration by power injection at 3.5 mL/sec. The DCE study consisted of five sequential acquisitions, with an interval of 30 s between them and acquisition times ranging from 10 s to 13 s. The first sequence began 21 s after the intravenous injection of contrast. An additional upper abdomen diffusion-weighted sequence was performed in order to identify distant metastases (to solid organs or lymph nodes). The MRI scans were analyzed by two radiologists, one specializing in MRI of the pelvis and the other specializing in MRI of the upper abdomen, who were working separately and were blinded to the histological diagnosis, as well as to the follow-up data. Both radiologists had vast prior experience (10 and 9 years, respectively) in the analysis of pelvic MRI scan. For all of the adnexal masses, each radiologist calculated the O-RADS MRI score independently. Disagreements regarding the final classification were resolved by consensus. O-RADS MRI score Adnexal masses were described with terms established in the literature and endorsed by the American College of Radiology(14,19), involving morphological features on MRI and the DCE standards necessary for applying the O-RADS MRI score. Table 1 illustrates the categories and main findings of the O-RADS MRI score. Statistical analysis Data were analyzed using the R Environment for Statistical Computing Software(22). We calculated the sensitivity, specificity, positive predictive value, and negative predictive value for O-RADS MRI scores, using ≥ 4 as the cutoff score for malignancy(17,18). For statistical purposes, borderline ovarian tumors were classified as malignant. RESULTS The histological subtypes of benign and malignant adnexal masses are shown in Table 2. A final histopathological diagnosis was made in 190 (66.20%) of the 287 masses evaluated in the present study. Because our hospital is a tertiary cancer center, the malignancy rate was high, 90 (47.37%) of those 190 masses being classified as malignant in the histopathological analysis. Of the remaining 97 masses, which were followed clinically, none showed signs of malignant transformation, maintaining an O-RADS MRI score of 2 (almost certainly benign) or 3 (low risk). Figure 2 illustrates an adnexal mass in the right ovary, with an O-RADS MRI score of 3 (low risk), which was resected surgically. In that case, the final histopathological diagnosis was benign mucinous cystadenoma.  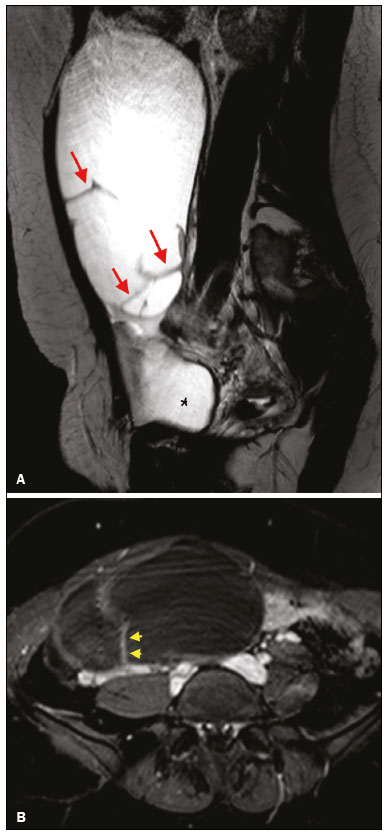 Figure 2. A 43-year-old woman with chronic pelvic pain and a right adnexal mass identified on ultrasound, with an indeterminate result based on the IOTA simple rules. A: T2-weighted sagittal sequence showing a cystic adnexal mass with multiple septa (red arrows) centered in the right adnexal region, near the bladder (asterisk). B: Contrast-enhanced axial fat-saturated T1-weighted sequence (acquisition at 30 s after contrast administration) showing enhancement of some septa (yellow arrowheads), with no solid portions. The final O-RADS MRI score was 3 (low risk). The patient underwent surgery (right oophorectomy), and the final histological diagnosis was mucinous cystadenoma. Table 3 shows the final O-RADS MRI score for all adnexal masses, together with the sensitivity, specificity, positive predictive value, negative predictive value, positive likelihood ratio, and negative likelihood ratio, with cutoff O-RADS MRI scores of 4 and 5 for malignancy. The O-RADS MRI score showed a sensitivity and specificity of 91.11% and 94.92%, respectively, with an overall accuracy of 93.73%. 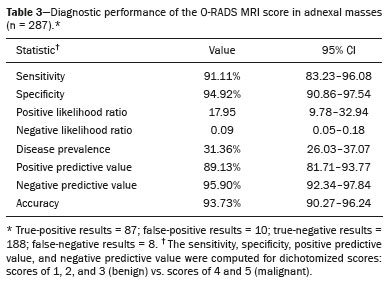 Table 4 shows the adnexal masses for which the O-RADS MRI score produced a false-positive or false-negative result, together with the key imaging findings responsible for the diagnostic error. Among the eight cases of false-negative results, there were five malignant masses that did not present an identifiable solid portion, which resulted in an O-RADS MRI score of 2 or 3, and three malignant masses with a solid component that had a low-risk (type 1) time-intensity curve, which resulted in an O-RADS MRI score of 3. Among the ten cases of false-positive results, there was a moderate-risk (type 2) time-intensity curve, resulting in an O-RADS MRI score of 4, in all of the masses. None of the masses presented a high-risk (type 3) time-intensity curve. 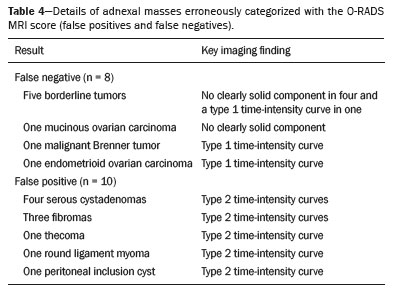 Figure 3 illustrates an adnexal mass in the left ovary, showing the visible enhancement pattern at 35 s after injection of the contrast and the high-risk (type 3) time-intensity curve. For that mass, the O-RADS MRI score was 5 and the final histological diagnosis was high-grade serous cystadenocarcinoma with peritoneal carcinomatosis. Figure 4 shows a solid-cystic mass, centered in the left adnexal region and extending to the upper abdomen, with lipid content and a large volume of enhancing solid tissue. The O-RADS MRI score for such masses is 4(18), and the final histological diagnosis in that case was immature teratoma accompanied by gliomatosis peritonei. 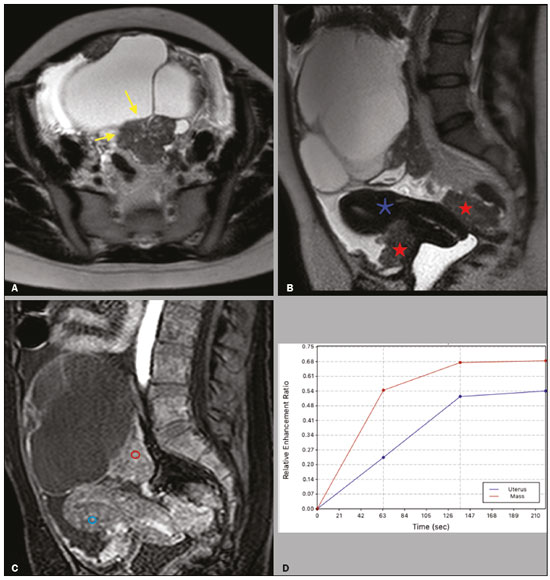 Figure 3. A 39 year-old woman with a family history of breast and ovarian cancer who presented with pelvic pain and a left adnexal mass. A: Axial T2-weighted sequence showing a multilocular cystic mass with solid portions (yellow arrows) centered in the left adnexal region. B: Axial T2-weighted sequence showing the multilocular cystic mass and multiple peritoneal implants (red stars) near the uterus (blue asterisk). C: Contrast-enhanced sagittal fat-saturated T1-weighted sequence (DCE study) showing visible enhancement of the solid component of the mass, greater than that of the myometrium, at 35 s after contrast administration. Note the region of interest over the uterus (blue circle) and the other over the adnexal mass (red circle). D: Relative enhancement ratio curve showing that the initial increase in the enhancement of the mass was greater than was that of the uterus. The final O-RADS MRI score was 5 (high risk). The patient underwent surgery, and the final histological diagnosis was high-grade serous cystadenocarcinoma with peritoneal carcinomatosis. 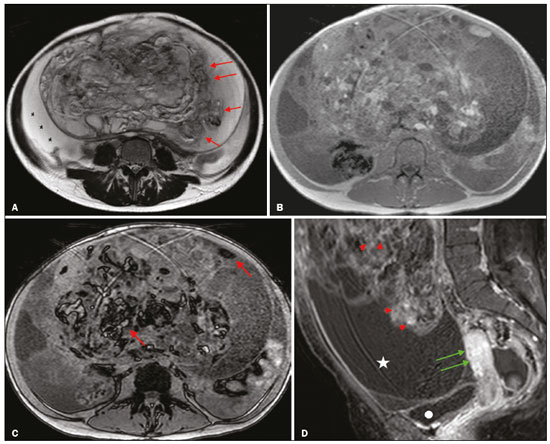 Figure 4. A 28-year-old woman with a left adnexal mass, extending to the upper abdomen, which had been considered indeterminate on ultrasound with the application of the IOTA simple rules. A: Axial T2-weighted sequence showing a solid-cystic mass (red arrows) centered in the left adnexal region. Note the ascites (asterisk). B,C: In-phase and out-of-phase T1-weighted sequences showing multiple foci of fat (signal drop in the out-of-phase sequence; red arrows), consistent with a germ cell tumor. D: Contrast-enhanced sagittal fat-saturated T1-weighted sequence (acquisition at 35 s after contrast administration) showing enhancement of the solid portions of the mass (red arrowheads) less than that of the myometrium (green arrows). Note the ascites (white star) and the location of the bladder (white circle). The final O-RADS MRI score was 4 (intermediate risk). The patient underwent surgery, and the final histological diagnosis was immature teratoma accompanied by gliomatosis peritonei. DISCUSSION The sensitivity, specificity, and overall accuracy of the O-RADS MRI score in the present study were similar to those reported for the original score(17), which further supports its use in the assessment of adnexal masses, especially those considered indeterminate on ultrasound. In the O-RADS MRI protocol, it is necessary to include sequences aimed at evaluating the morphology of the adnexal mass—at least two T2-weighted multiplanar sequences (axial and sagittal or coronal) and T1-weighted sequences, with and without fat saturation, in order to stratify fat and blood components—and advanced sequences—diffusion-weighted sequences with b values of 800–1200 s/mm2 (enough b to suppress the T2 shine-through effect, thus ensuring that the urine in the bladder is black) and a DCE study, with a time resolution ≤ 15 s and a total time after gadolinium injection of 180 s. If DCE is unavailable (because of limitations of the magnet or software), the contrast uptake can be analyzed visually (at 30–40 s after gadolinium injection). In addition, the use of gadolinium can be foregone if no suspicious adnexal lesion is identified in the analysis of the conventional (T1- and T2-weighted) sequences, such as when only follicles or the corpus luteum are identified in a premenopausal patient. The contrast uptake (DCE) study is the cornerstone of the O-RADS MRI score, defining the cutoff scores of 4 and 5. A moderate or marked increase in the signal intensity of an ovarian mass, in relation to that of the uterus, after gadolinium injection is associated with borderline and malignant tumors, correlating directly with the angiogenic status of a tumor(23–25). In the initial study of the ADNEX MR SCORING system(14), the DCE study images were obtained sequentially at intervals of 2.4 s, starting from 10 s after injection of the contrast, over a total of 320 s, with consequent post-processing on a workstation. That technical requirement was very rigid and complex, which could limit its use in clinical practice, a difficulty acknowledged by the authors(15). The O-RADS MRI score allows the use of lower temporal resolution in the DCE study, with intervals of ≤ 15 s and a total acquisition time of 180 s, as well as including the option of performing a comparative visual analysis between the pattern of enhancement of the adnexal mass and that of the myometrium (at 30–40 s after gadolinium injection) when a DCE study is not available(18). Those changes will allow the dissemination of the O-RADS MRI score to a greater number of MRI diagnostic centers, even those using low-field scanners, which is still a reality in low- and middle-income countries(26). Another update was the classification of adnexal masses with lipid content and a large volume of enhancing solid tissue as deserving of an O-RADS MRI score of 4(18). That is important because some malignant tumors with a fat component, such as immature teratomas, can mimic benign lesions and need to be carefully evaluated(27). However, minimal enhancement of Rokitansky nodules in a lesion containing lipid does not change the classification to an O-RADS MRI score of 4(18). Therefore, according to the O-RADS MRI scoring system, not every adnexal mass with fat content is benign, especially in women under 20 years of age, with or without changes in serum biomarkers, lactic dehydrogenase, and alpha-fetoprotein(28,29). Certain diagnostic challenges persist, especially in cases of borderline tumors or invasive malignant tumors with type 1 time-intensity curves or without clearly solid portions, which were the main causes of the false-negative O-RADS MRI score results in the present study. The use of new diagnostic parameters and imaging concepts, especially radiogenomics studies(30) and tailored imaging protocols for borderline malignancy(31), could facilitate the stratification of such tumors. Our study has some limitations. First, it was conducted at a tertiary cancer center, which increased the positive predictive value for malignancy in our sample. In addition, MRI reporting was performed by experienced examiners, whereas it is recommended that the reporting be performed by less experienced examiners or generalist radiologists. Furthermore, we did not assess the accuracy of the use of a single analysis of enhancement at 30–40 s after gadolinium injection in determining O-RADS MRI scores of 4 and 5, which could have altered our findings. Other authors have even evaluated the use of unenhanced images to construct the O-RADS MRI score, especially when clinical conditions, such as nephropathy or severe allergy(32), preclude the use of contrast. In summary, our data support the use of the O-RADS MRI score to assess adnexal masses, especially those considered indeterminate on ultrasound. The latest updates to the O-RADS MRI score facilitate its interpretation and will allow its use to become more widespread, with no loss of diagnostic accuracy. REFERENCES 1. American College of Obstetricians and Gynecologists’ Committee on Practice Bulletins–Gynecology. Practice Bulletin No. 174: evaluation and management of adrenal masses. Obstet Gynecol. 2016;128:e210–e226. 2. Pickhardt PJ, Hanson ME. Incidental adnexal masses detected at low-dose unenhanced CT in asymptomatic women age 50 and older: implications for clinical management and ovarian cancer screening. Radiology. 2010;257:144–50. 3. Thakur M, Timmerman D. Imaging of adnexal masses. Clin Obstet Gynecol. 2017;60:38–45. 4. McDonald JM, Modesitt SC. The incidental postmenopausal adnexal mass. Clin Obstet Gynecol. 2006;49:506–16. 5. International Agency for Research on Cancer. World Health Organization. Ovary. [cited 2021 March 9]. Available from: https://gco.iarc.fr/today/data/factsheets/cancers/25-Ovary-fact-sheet.pdf. 6. Woo YL, Kyrgiou M, Bryant A, et al. Centralisation of services for gynaecological cancers – a Cochrane systematic review. Gynecol Oncol. 2012;126:286–90. 7. Timmerman D, Valentin L, Bourne TH, et al. Terms, definitions and measurements to describe the sonographic features of adnexal tumors: a consensus opinion from the International Ovarian Tumor Analysis (IOTA) Group. Ultrasound Obstet Gynecol. 2000;16:500–5. 8. Timmerman D, Testa AC, Bourne T, et al. Simple ultrasound-based rules for the diagnosis of ovarian cancer. Ultrasound Obstet Gynecol. 2008;31:681–90. 9. Sadowski EA, Rockall AG, Maturen KE, et al. Adnexal lesions: imaging strategies for ultrasound and MR imaging. Diagn Interv Imaging. 2019;100:635–46. 10. Kaijser J, Vandecaveye V, Deroose CM, et al. Imaging techniques for the pre-surgical diagnosis of adnexal tumours. Best Pract Res Clin Obstet Gynaecol. 2014;28:683–95. 11. Jacobs IJ, Menon U, Ryan A, et al. Ovarian cancer screening and mortality in the UK Collaborative Trial of Ovarian Cancer Screening (UKCTOCS): a randomised controlled trial. Lancet. 2016;387:945–56. 12. Forstner R, Thomassin-Naggara I, Cunha TM, et al. ESUR recommendations for MR imaging of the sonographically indeterminate adnexal mass: an update. Eur Radiol. 2017;27:2248–57. 13. Anthoulakis C, Nikoloudis N. Pelvic MRI as the “gold standard” in the subsequent evaluation of ultrasound-indeterminate adnexal lesions: a systematic review. Gynecol Oncol. 2014;132:661–8. 14. Thomassin-Naggara I, Aubert E, Rockall A, et al. Adnexal masses: development and preliminary validation of an MR imaging scoring system. Radiology. 2013;267:432–43. 15. Sadowski EA, Robbins JB, Rockall AG, et al. A systematic approach to adnexal masses discovered on ultrasound: the ADNEx MR scoring system. Abdom Radiol (NY). 2018;43:679–95. 16. Pereira PN, Sarian LO, Yoshida A, et al. Accuracy of the ADNEX MR scoring system based on a simplified MRI protocol for the assessment of adnexal masses. Diagn Interv Radiol. 2018;24:63–71. 17. Thomassin-Naggara I, Poncelet E, Jalaguier-Coudray A, et al. Ovarian-Adnexal Reporting Data System Magnetic Resonance Imaging (O-RADS MRI) score for risk stratification of sonographically indeterminate adnexal masses. JAMA Netw Open. 2020;3:e1919896. 18. American College of Radiology. O-RADS MRI risk score governing concepts: O-RADS MRI risk stratification and management system. [cited 2021 March 9]. Available from: https://www.acr.org/-/media/ACR/Files/RADS/O-RADS/O-RADS-MR-Risk-Stratification-System-Table-September-2020.pdf. 19. American College of Radiology. Ovarian-Adnexal Reporting & Data System (O-RADS). [cited 2021 March 9]. Available from: https://www.acr.org/Clinical-Resources/Reporting-and-Data-Systems/O-Rads#MRI. 20. D’Orsi CJ, Sickles EA, Mendelson EB, et al. ACR BI-RADS® Atlas – Breast Imaging Reporting and Data System. Reston, VA: American College of Radiology; 2013. 21. Kurman RJ, Carcangiu ML, Herrington CS, et al. WHO classification of tumours of female reproductive organs. 4th edition. Lyon, France: IARC Publications; 2014. 22. R Core Team. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2014. 23. Dilks P, Narayanan P, Reznek R, et al. Can quantitative dynamic contrast-enhanced MRI independently characterize an ovarian mass? Eur Radiol. 2010;20:2176–83. 24. Thomassin-Naggara I, Daraï E, Cuenod CA, et al. Dynamic contrast-enhanced magnetic resonance imaging: a useful tool for characterizing ovarian epithelial tumors. J Magn Reson Imaging. 2008;28:111–20. 25. Thomassin-Naggara I, Bazot M, Daraï E, et al. Epithelial ovarian tumors: value of dynamic contrast-enhanced MR imaging and correlation with tumor angiogenesis. Radiology. 2008;248:148–59. 26. Ogbole GI, Adeyomoye AO, Badu-Peprah A, et al. Survey of magnetic resonance imaging availability in West Africa. Pan Afr Med J. 2018;30:240. 27. Shaaban AM, Rezvani M, Elsayes KM, et al. Ovarian malignant germ cell tumors: cellular classification and clinical and imaging features. Radiographics. 2014;34:777–801. 28. Heo SH, Kim JW, Shin SS, et al. Review of ovarian tumors in children and adolescents: radiologic-pathologic correlation. Radiographics. 2014;34:2039–55. 29. Shinkai T, Masumoto K, Chiba F, et al. Pediatric ovarian immature teratoma: histological grading and clinical characteristics. J Pediatr Surg. 2020;55:707–10. 30. Nougaret S, McCague C, Tibermacine H, et al. Radiomics and radiogenomics in ovarian cancer: a literature review. Abdom Radiol (NY). 2021;46:2308–22. 31. Li YA, Qiang JW, Ma FH, et al. MRI features and score for differentiating borderline from malignant epithelial ovarian tumors. Eur J Radiol. 2018;98:136–42. 32. Sahin H, Panico C, Ursprung S, et al. Non-contrast MRI can accurately characterize adnexal masses: a retrospective study. Eur Radiol. 2021;31:6962–73. 1. Department of Obstetrics and Gynecology, Faculdade de Ciências Médicas da Universidade Estadual de Campinas (FCM-Unicamp), Campinas, SP, Brazil 2. Department of Radiology, Hospital das Clínicas da Faculdade de Ciências Médicas da Universidade Estadual de Campinas (FCM-Unicamp), Campinas, SP, Brazil a. https://orcid.org/0000-0002-4621-8537 b. https://orcid.org/0000-0002-5982-0623 c. https://orcid.org/0000-0002-9554-6131 d. https://orcid.org/0000-0001-7542-9184 e. https://orcid.org/0000-0002-8802-8870 f. https://orcid.org/0000-0003-1029-9993 Correspondence: Dr. Patrick Nunes Pereira Departamento de Tocoginecologia – FCM-Unicamp Rua Tessália Vieira de Camargo, 126, Cidade Universitária Campinas, SP, Brazil, 13083-887 Email: patricknunes@gmail.com Received 19 March 2021 Accepted after revision 5 August 2021 Publication date: 09/12/2021 |
|
Av. Paulista, 37 - 7° andar - Conj. 71 - CEP 01311-902 - São Paulo - SP - Brazil - Phone: (11) 3372-4544 - Fax: (11) 3372-4554