Radiologia Brasileira - Publicação Científica Oficial do Colégio Brasileiro de Radiologia
AMB - Associação Médica Brasileira CNA - Comissão Nacional de Acreditação
 Vol. 53 nº 2 - Mar. / Apr. of 2020
Vol. 53 nº 2 - Mar. / Apr. of 2020
|
REVIEW ARTICLE
|
|
Ultrasound versus electromyography for the detection of fasciculation in amyotrophic lateral sclerosis: systematic review and meta-analysis |
|
|
Autho(rs): Márcio Luís Duarte1,a; Wagner Iared1,b; Acary Souza Bulle Oliveira1,c; Lucas Ribeiro dos Santos2,d; Maria Stella Peccin1,e |
|
|
Keywords: Ultrasonography; Electromyography; Fasciculation; Amyotrophic lateral sclerosis. |
|
|
Abstract: INTRODUCTION
Fasciculations are rapid, random, fine, flickering, or vermicular twitching movements of a group of muscle fibers innervated by a single motor unit(1–7). Although fasciculation is almost obligatory among patients with amyotrophic lateral sclerosis (ALS), which is the most common motor neuron disease and the one with the highest mortality, it can also occur in other diseases and conditions(1,2,4–6,8–12):
Fasciculation is a clinical and electromyographic marker of ALS, particularly when it is generalized and is accompanied by muscle loss or electromyographic changes indicative of denervation(13,14). Fasciculation may be detected by clinical evaluation, electromyography (EMG), or ultrasound(15–17). As diagnostic methods, EMG and ultrasound each have advantages and disadvantages. However, there is as yet no screening protocol for either method. Currently, EMG is the gold standard for assessing LMN function in ALS(1,18). For the diagnosis of LMN disease, fasciculation potentials should be detected in several regions(1). The sensitivity of EMG is dependent on the duration of screening of each muscle and the number of muscles evaluated(15,19). Ultrasound is highly sensitive to movement, allowing good visualization of fasciculation(1,19,20). Minimal movements as small as 5 µm are detectable, and the temporal resolution (frame rate) is more than 80 fps(1). Neither computed tomography nor magnetic resonance imaging has that advantage, because they are static exams(18). Studies have shown that EMG assesses only the superficial musculature, is limited in terms of the area it can study, is not capable of evaluating atrophy, and takes 10–90 s to detect a fasciculation(1,21–23). In contrast, ultrasound assesses the superficial and deep musculature, thereby allowing a greater number of motor units to be studied(24,25), is able to identify atrophy, as well as to calculate the cross-sectional area of evaluation, and takes only 8–10 s to detect a fasciculation(1,21,22). Ultrasound is a noninvasive, painless method that is more widely available than is EMG, as well as being less expensive and not involving the use of radiation(1,12,14,26–31). However, ultrasound does not differentiate between benign (stable) and malignant (unstable) fasciculations. That differentiation is made by using EMG to assess motor unit potentials(22,32). There is currently no one diagnostic modality capable of detailing all events occurring in a muscle or muscle group over time(1). The objectives of this study were to determine the accuracy of ultrasound and EMG for the detection of fasciculation, to compare the rate of fasciculation detection using ultrasound and EMG in patients with ALS, to determine which muscles are better assessed with ultrasound and EMG, and to evaluate the ability of ultrasound to identify muscle atrophy. METHOD This was a systematic review of studies of diagnostic accuracy, as defined in the Cochrane Handbook for Systematic Reviews of Diagnostic Test Accuracy, version 5.1. Studies evaluating the diagnostic accuracy of ultrasound and EMG for the detection of fasciculation were included regardless of publication status. There was no language restriction. The study was approved by the local institutional review board, and the review was previously registered with the International Prospective Register of Systematic Reviews (Registration no. CRD42017078388). From reference journals, we selected relevant articles or abstracts that were deemed potentially eligible for inclusion. Two authors, working independently, identified the eligible texts. In cases of disagreement, a third author was consulted. Data were extracted through the use of a standardized form. Eligible studies with a control group were evaluated using a quality assessment tool, the Quality Assessment of Diagnostic Accuracy Studies, version 2(33). In all eligible studies, including those with a control group, we used the RTI item bank questionnaire, which is a tool focused on the evaluation of biases and precision(34,35). We searched the Cochrane Library, MEDLINE, Excerpta Medica, and Latin-American and Caribbean Health Sciences Literature databases for studies published up through January of 2019. We also evaluated the bibliographies of the included studies and the main review articles on the subject. Manual searches of the bibliographies were also conducted. For all analyses and diagrams, we employed the software Review Manager, version 5.3 (RevMan 5; Cochrane Collaboration, Oxford, UK) and Meta-DiSc, version 1.4 (Cochrane Colloquium, Barcelona, Spain). Studies selected We identified 139 studies on the subject and selected 12 that met the inclusion criteria. Two of those studies were excluded: one because ultrasound and EMG were performed at the same time, so the study was not blinded(36); another because it did not contain all the necessary data(18). Therefore, ten studies were included in the review and meta-analysis(15,21,26,37–43). Of those ten studies, six included a control group, allowing assessment of accuracy. The four studies that did not include a control group were included only in the analysis of the detection rate for each method. Among the ten studies included, the duration of the ultrasound examination of each muscle was 10 s in four studies and 30 s in another four, whereas one study did not report the duration and both durations were employed in one study. For the last study, we separated the data in our analysis of the detection rates by ultrasound regardless of examination time, so that the patients who underwent the exam at both durations were not counted twice. We utilized the 30-s data for those patients, because all of the muscles that presented fasciculation within 10 s also presented fasciculation within 30 s. Data from the study that did not mention the duration of the ultrasound examination were used only in the analysis of the detection rates. In five studies, each muscle was evaluated individually, allowing us to evaluate the specific detection rate for each muscle. In all muscles, the rate of fasciculation detection was higher for ultrasound than for EMG, independent of the duration of the ultrasound examination. In the analysis of the detection rates in patients, that difference was even greater. For individual muscles and for patients, Tables 1, 2, and 3 compare the two methods at ultrasound examination times of 10 s, 30 s, and both, respectively. The forest plots in Figures 1 and 2 separate those data for muscles and for patients, respectively. 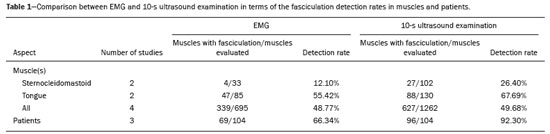 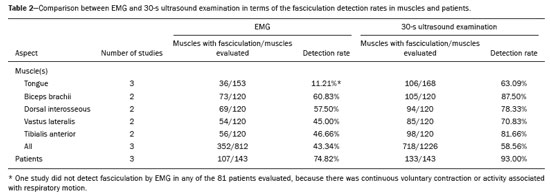 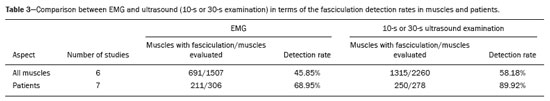 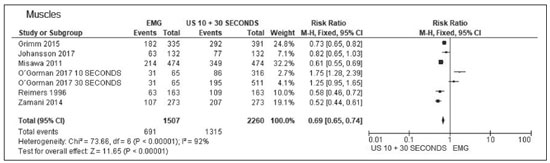 Figure 1. Forest plot. Comparison of EMG and ultrasound (10-s and 30-s examinations) in muscles. 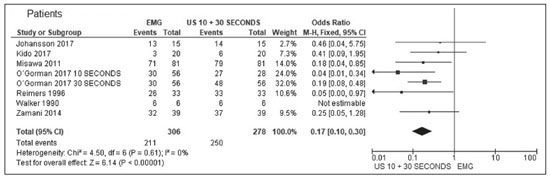 Figure 2. Forest plot. Comparison of EMG and ultrasound (10-s and 30-s examinations) in patients. Accuracy assessment Two studies had a control group that described the number of muscles, thus allowing us to evaluate accuracy. Both studies used ultrasound examination times of 10 s. Five studies had a control group and reported the number of patients, thus allowing us to assess 10-s ultrasound accuracy. Two studies had a control group and reported the number of muscles, thus allowing us to evaluate accuracy. Both studies used ultrasound examination times of 30 s. In two randomized clinical trials(38,40), the 10-s ultrasound examination showed a sensitivity of 76% and a specificity of 73% for the detection of fasciculations in ALS, with a 95% confidence interval p < 0.05 and an accuracy of 70%. In five other randomized clinical trials(15,37,38,40,41), the 10-s ultrasound examination showed a sensitivity of 75% and a specificity of 93% for the detection of fasciculations in ALS, with a 95% confidence interval p < 0.05 and an accuracy of 85%. In two other randomized clinical trials(40,43), the 30-s ultrasound examination showed a sensitivity of 94% and a specificity of 80% for the detection of fasciculations in ALS, with a 95% confidence interval p < 0.05 and an accuracy of 82%. Therefore, in all examinations, the accuracy was higher than 70%. DISCUSSION We found ultrasound to be the method with the best accuracy for detection of fasciculation, as previously reported in the medical literature(26,36). The method is accurate for evaluating patients and for evaluating individual muscles, regardless of the duration of the examination. The difference between the 10- and 30-s ultrasound examinations for the detection of fasciculation was not significant, although the 30-s examinations provided more data for each muscle, allowing the analysis of greater numbers of patients and muscles. Ultrasound had a sensitivity and specificity higher than 70% for both evaluations (individual muscles and patients). The specificity was higher than 90% in the patients evaluated by ultrasound. The muscles for which ultrasound presented the best detection rate (%) were the biceps brachii (88%) and the tibialis anterior (82%), both during 30-s examinations, followed by the vastus lateralis (71%), also during 30-s examinations. During the 10-s examinations, the detection rate was highest (68%) for the tongue muscle and lowest (26%) for the sternocleidomastoid muscle. The best detection rates achieved with EMG were for the biceps brachii muscles (61%) and the dorsal interosseous muscles (58%). The technique of detecting fasciculation by ultrasound is easily learned, as evidenced by the level of interobserver agreement reported—100% for the presence or absence of fasciculations(20,29,36,43,44). Ultrasound identifies fasciculations in 80% of cases, compared with only 45% for intramuscular EMG(1). That could be explained by the fact that EMG is able to evaluate only a few motor units, many fewer than those evaluated by ultrasound(41). Fasciculations in specific muscle groups, such as those of the arm and trunk, as well as in the sartorius and tibialis anterior muscles, are of much greater diagnostic significance than are those detected in the quadriceps, calves, or hamstrings(41). Fasciculations are more common in the distal muscles of the leg than elsewhere in the body(42). It should be borne in mind that EMG evaluation of the tongue is quite limited because it is difficult to achieve complete relaxation of the tongue muscle(45–47), as confirmed by Misawa et al.(21), who were unable to detect fasciculation by EMG in the tongues of 81 patients evaluated, suggesting that ultrasound is more feasible for that task(21). Ultrasound and EMG both have disadvantages. There is as yet no single modality that provides all of the details of events that occur within a muscle or muscle group over a given period of time(1). Ultrasound is a practical, painless technique that can allow earlier diagnosis and provide greater confidence in the diagnosis of ALS, due to the visualization of fasciculations(21). Ultrasound is also valuable in making the differential diagnosis with similar diseases(22). Musculoskeletal ultrasound may add knowledge to neurophysiological test data by detecting changes in muscle morphology and echotexture that can imply denervation(30,31). Ultrasound can also be utilized as a screening tool before submitting patients to procedures that are more invasive and painful, such as EMG and muscle biopsy(48,49). The advantage of ultrasound over EMG is its greater sensitivity in detecting fasciculations(37). According to Grimm et al.(37), the disadvantage of ultrasound is its inability to distinguish between stable and unstable fasciculations, which may be additional criteria for the diagnosis of motor neuron diseases. Currently, the distinction between stable and unstable fasciculations can be made only with EMG. In another study, Grimm et al.(49) suggested that ultrasound is also able to detect muscle fasciculation in the early stages of sepsis. Extensive involvement of the arm muscles over time may be a sign of sepsis-induced muscle disorders(49). That fact, together with the greater accessibility of ultrasound and its accuracy in the detection of fasciculation, may point to a new direction in its indications for uses other than the evaluation of LMN diseases, especially ALS, in which ultrasound may soon replace EMG as the best diagnostic method. Given the high sensitivity of ultrasound for the detection of fasciculation, the method can contribute to the evaluation of this sign in other diseases, including metabolic diseases (hyperparathyroidism, hyperthyroidism, and hypomagnesemia) and conditions induced by the use of drugs (anticholinesterases, caffeine, lithium, theophylline, and terbutaline). Ultrasound has all of the necessary characteristics to be used as an ALS screening method, including low cost, availability, accessibility, high sensitivity, and high specificity, as well as being painless(43). According to our findings in this review, ultrasound is suggested as a screening method for the evaluation of fasciculation in all motor neuron diseases, including ALS, thereby improving the rate of fasciculation detection and significantly reducing diagnostic costs. In this systematic review, we have identified the need for new prospective clinical trials to evaluate muscles not yet studied as well as those for which there are no definitive results, such as the rectus femoris muscle, which has been evaluated in only one clinical trial(37). The muscles for which the detection rates in the 30-s ultrasound examinations were highest were the biceps brachii and the tibialis anterior muscles. Ultrasound can detect muscle atrophy, fatty infiltration, and intramuscular fibrosis(49). However, in our searches, we identified no studies evaluating the use of ultrasound for the detection of atrophy in ALS. CONCLUSION The importance of this systematic review is that we have shown ultrasound to be a more accurate method for the detection of fasciculation than is EMG. In comparison with EMG, ultrasound is more affordable and accessible, as well as being noninvasive, being able to evaluate more muscle fibers, and providing information on muscle atrophy. REFERENCES 1. Harding PJ, Loram ID, Combes N, et al. Ultrasound-based detection of fasciculations in healthy and diseased muscles. IEEE Trans Biomed Eng. 2016;63:512–8. 2. Buainain R, Moura LS, Oliveira ASB. Fasciculação. Rev Neurociências. 2000;8:31–4. 3. Simon NG. Dynamic muscle ultrasound – another extension of the clinical examination. Clin Neurophysiol. 2015;126:1466–7. 4. Takamatsu N, Nodera H, Mori A, et al. Which muscle shows fasciculations by ultrasound in patients with ALS? J Med Invest. 2016; 63:49–53. 5. Wenzel S, Scheel AK, Reimers CD. Faszikulationen – Überblick über Pathophysiologic, klinische Bedeutung und Nachweis. Klin Neurophysiol. 1998;29:271–9. 6. Wenzel S, Herrendorf G, Scheel A, et al. Surface EMG and myosonography in the detection of fasciculations: a comparative study. J Neuroimaging. 1998;8:148–54. 7. Scheel AK, Reimers CD. Detection of fasciculations and other types of muscular hyperkinesias with ultrasound. Ultraschall Med. 2004;25:337–41. 8. Di L, Chen H, Da Y, et al. Atypical familial amyotrophic lateral sclerosis with initial symptoms of pain or tremor in a Chinese family harboring VAPB-P56S mutation. J Neurol. 2016;263:263–8. 9. Joyce NC, Carter GT. Electrodiagnosis in persons with amyotrophic lateral sclerosis. PMR. 2013;5(5 Suppl):S89–95. 10. Dimberg EL. The office evaluation of weakness. Semin Neurol. 2011; 31:115–30. 11. Oliveira ASB, Pereira RDB. Amyotrophic lateral sclerosis (ALS): three letters that change the people’s life. For ever. Arq Neuropsiquiatr. 2009;67:750–82. 12. Tsuji Y, Noto YI, Shiga K, et al. A muscle ultrasound score in the diagnosis of amyotrophic lateral sclerosis. Clin Neurophysiol. 2017; 128:1069–74. 13. Noto YI, Shibuya K, Shahrizaila N, et al. Detection of fasciculations in amyotrophic lateral sclerosis: the optimal ultrasound scan time. Muscle Nerve. 2017;56:1068–71. 14. Noto YI, Simon NG, Selby A, et al. Ectopic impulse generation in peripheral nerve hyperexcitability syndromes and amyotrophic lateral sclerosis. Clin Neurophysiol. 2018;129:974–80. 15. Arts IM, Overeem S, Pillen S, et al. Muscle ultrasonography: a diagnostic tool for amyotrophic lateral sclerosis. Clin Neurophysiol. 2012;123:1662–7. 16. Osaki Y, Takamatsu N, Shimatani Y, et al. Ultrasonographic evaluation of myokymic discharges. Clin Neurophysiol. 2015;126:1638– 9. 17. Cartwright MS. Neuromuscular ultrasound for the evaluation of amyotrophic lateral sclerosis [dissertation]. Winston-Salem, NC: Wake Forest University; 2012. 18. Arts IM, van Rooij FG, Overeem S, et al. Quantitative muscle ultrasonography in amyotrophic lateral sclerosis. Ultrasound Med Biol. 2008;34:354–61. 19. Deroide N, Bousson V, Lévy BI, et al. Nerve and muscle imaging in peripheral neuropathy associated to electroneuromyography: the ideal couple? Rev Med Interne. 2010;31:287–94. 20. Pillen S, Van Alfen N. Muscle ultrasound from diagnostic tool to outcome measure—quantification is the challenge. Muscle Nerve. 2015;52:319–20. 21. Misawa S, Noto Y, Shibuya K, et al. Ultrasonographic detection of fasciculations markedly increases diagnostic sensitivity of ALS. Neurology. 2011;77:1532–7. 22. Carvalho M, Swash M. Lower motor neuron dysfunction in ALS. Clin Neurophysiol. 2016;127:2670–81. 23. Kleine BU, Boekestein WA, Arts IM, et al. Fasciculations and their F-response revisited: high-density surface EMG in ALS and benign fasciculations. Clin Neurophysiol. 2012;123:399–405. 24. Katzberg HD, Bril V, Breiner A. Ultrasound in neuromuscular disorders. J Clin Neurophysiol. 2016;33:80–5. 25. Rico-Santos M, Segura T. The value of ultrasounds in the detection of muscle fasciculation. Rev Neurol. 2013;56:589. 26. Walker FO, Donofrio PD, Harpold GJ, et al. Sonographic imaging of muscle contraction and fasciculations: a correlation with electromyography. Muscle Nerve. 1990;13:33–9. 27. Pillen S, van Alfen N. Skeletal muscle ultrasound. Neurol Res. 2011; 33:1016–24. 28. Simon NG, Ralph JW, Lomen-Hoerth C, et al. Quantitative ultrasound of denervated hand muscles. Muscle Nerve. 2015;52:221– 30. 29. Tremolizzo L, Susani E, Aliprandi A, et al. Muscle ultrasonography for detecting fasciculations in frontotemporal dementia. Amyotroph Lateral Scler Frontotemporal Degener. 2014;15:546–50. 30. van Alfen N, Gijsbertse K, de Korte CL. How useful is muscle ultrasound in the diagnostic workup of neuromuscular diseases? Curr Opin Neurol. 2018;31:568–74. 31. van Alfen N, Mah JK. Neuromuscular ultrasound: a new tool in your toolbox. Can J Neurol Sci. 2018;45:504–15. 32. Carvalho M. Pathophysiological significance of fasciculations in the early diagnosis of ALS. Amyotroph Lateral Scler Other Motor Neuron Disord. 2000;1 Suppl 1:S43–6. 33. Whiting PF, Rutjes AW, Westwood ME, et al. QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med. 2011;155:529–36. 34. Margulis AV, Pladevall M, Riera-Guardia N, et al. Quality assessment of observational studies in a drug-safety systematic review, comparison of two tools: the Newcastle-Ottawa Scale and the RTI item bank. Clin Epidemiol. 2014;6:359–68. 35. Viswanathan M, Berkman ND. Development of the RTI item bank on risk of bias and precision of observational studies. J Clin Epidemiol. 2012;65:163–78. 36. Regensburger M, Tenner F, Möbius C, et al. Detection radius of EMG for fasciculations: empiric study combining ultrasonography and electromyography. Clin Neurophysiol. 2018;129:487–93. 37. Grimm A, Prell T, Décard BF, et al. Muscle ultrasonography as an additional diagnostic tool for the diagnosis of amyotrophic lateral sclerosis. Clin Neurophysiol. 2015;126:820–7. 38. Johansson MT, Ellegaard HR, Tankisi H, et al. Fasciculations in nerve and muscle disorders – a prospective study of muscle ultrasound compared to electromyography. Clin Neurophysiol. 2017; 128:2250–7. 39. Kido M, Fujisaki N, Miyagi T, et al. Complimentary use of needle electromyography and ultrasonography of tongue is effective for early detection of abnormality in 20 patients with amyotrophic lateral sclerosis. Rinsho Shinkeigaku. 2017;57:681–4. 40. O’gorman CM, Weikamp JG, Baria M, et al. Detecting fasciculations in cranial nerve innervated muscles with ultrasound in amyotrophic lateral sclerosis. Muscle Nerve. 2017;56:1072–6. 41. Reimers CD, Ziemann U, Scheel A, et al. Fasciculations: clinical, electromyographic, and ultrasonographic assessment. J Neurol. 1996; 243:579–84. 42. Zamani B, Rohani M, Emami K, et al. The accuracy of direct ultrasound study of the muscles in diagnosis of ALS comparing to electromyography in patients refferred to Rasoul Akram hospital during 2012-2013 [abstract poster]. Cerebrovasc Dis. 2014;37(Suppl 2):55. 43. Krämer HH, Vlazak A, Döring K, et al. Excellent interrater agreement for the differentiation of fasciculations and artefacts – a dynamic myosonography study. Clin Neurophysiol. 2014;125:2441–5. 44. Hobson-Webb LD, Padua L. Small steps … and leaps … toward big science: multicenter studies in neuromuscular ultrasound. Clin Neurophysiol. 2014;125:2326–7. 45. Sonoo M, Kuwabara S, Shimizu T, et al. Utility of trapezius EMG for diagnosis of amyotrophic lateral sclerosis. Muscle Nerve. 2009; 39:63–70. 46. Li J, Petajan J, Smith G, et al. Electromyography of sternocleidomastoid muscle in ALS: a prospective study. Muscle Nerve. 2002; 25:725–8. 47. Finsterer J, Erdorf M, Mamoli B, et al. Needle electromyography of bulbar muscles in patients with amyotrophic lateral sclerosis: evidence of subclinical involvement. Neurology. 1998;51:1417–22. 48. Misawa S. Utility of muscle ultrasonography for the diagnosis of amyotrophic lateral sclerosis. Brain Nerve. 2014;66:229–36. 49. Grimm A, Teschner U, Porzelius C, et al. Muscle ultrasound for early assessment of critical illness neuromyopathy in severe sepsis. Crit Care. 2013;17:R227. 1. Escola Paulista de Medicina da Universidade Federal de São Paulo (EPM-Unifesp), São Paulo, SP, Brazil 2. Centro Universitário Lusíada (Unilus) – Fundação Lusíada, São Paulo, SP, Brazil a. http://orcid.org/0000-0002-7874-9332 b. http://orcid.org/0000-0002-6426-5636 c. http://orcid.org/0000-0002-6986-4937 d. http://orcid.org/0000-0001-7897-1198 e. http://orcid.org/0000-0003-0329-4588 Correspondence: Dr. Wagner Iared EPM-Unifesp – Programa de Pós-Graduação em Saúde Baseada em Evidências Rua Napoleão de Barros, 865, Vila Clementino São Paulo, SP, Brazil, 04024-002 Email: wagneriared@gmail.com Received 8 April 2019 Accepted after revision 21 June 2019 |
|
Av. Paulista, 37 - 7° andar - Conj. 71 - CEP 01311-902 - São Paulo - SP - Brazil - Phone: (11) 3372-4544 - Fax: (11) 3372-4554