Radiologia Brasileira - Publicação Científica Oficial do Colégio Brasileiro de Radiologia
AMB - Associação Médica Brasileira CNA - Comissão Nacional de Acreditação
 Vol. 52 nº 3 - May / June of 2019
Vol. 52 nº 3 - May / June of 2019
|
ORIGINAL ARTICLE
|
|
Detection of additional primary malignancies: the role of CT and PET/CT combined with multiple percutaneous biopsy |
|
|
Autho(rs): Tiago Kojun Tibana1; Rômulo Florêncio Tristão Santos2; Adalberto Arão Filho3; Bernardo Bacelar4; Leticia de Assis Martins5; Rafael Oliveira de Souza6; Edson Marchiori7; Thiago Franchi Nunes8 |
|
|
Keywords: Neoplasms, second primary/etiology; Biopsy, needle/methods; Positron-emission tomography/methods; Tomography, X-ray computed/methods; Fluorodeoxyglucose F18. |
|
|
Abstract: INTRODUCTION
Multiple primary tumors can be defined as more than one, histologically different, synchronous or metachronous lesion in the same individual(1). Although uncommon, their incidence and prevalence have been progressively increasing, due in large part to the increase in life expectancy of the population and to advances in diagnostic techniques(2). From a diagnostic point of view, their early recognition and confirmation are essential to achieving the ideal treatment. Therefore, radiologists should be familiar with the different patterns of presentation in patients with multiple primary tumors(2). Conventional imaging modalities, including ultrasound, computed tomography (CT), and magnetic resonance imaging (MRI), have limitations in the detection of multiple tumors, due to their regional imaging standard. In recent years, 18F-fluorodeoxyglucose positron emission tomography/computed tomography (18F-FDG PET/CT) has emerged as a promising imaging modality in the evaluation of malignant tumors(3). In addition, studies indicate that the introduction of 8F-FDG PET/CT images to evaluate malignant tumors results in better detection of multiple primary tumors(4,5). The advent of interventional radiology made possible notable advances in the diagnosis and treatment of various situations. Because of ongoing improvements in imaging methods and the need to seek more effective and less aggressive treatments, image-guided procedures performed by interventional radiologists have taken on added importance in the field of oncology(6). The objective of this study was to evaluate the imaging findings of 18F-FDG PET/CT and CT in patients with additional primary tumors, correlating the results with those of the method used in order to elucidate the diagnosis and of the pathology reports. MATERIALS AND METHODS We retrospectively analyzed the images acquired at two imaging and interventional radiology clinics, one at a public hospital and another at a private clinic, between January 2016 and January 2018. Clinical data were obtained from medical records and through telephone contact with physicians, patients, and family members. We selected patients who had presented with histologically proven synchronous or metachronous primary tumors and had undergone CT and/or PET/CT for either diagnostic or follow-up purposes. The inclusion criterion was the presence of at least two neoplasms, confirmed by histopathological examination, with distinctive histopathology at the different sites. We used an interval of six months to differentiate between synchronous and metachronous neoplasms, a criterion previously used by several other authors(7-9). Patients in whom there was no diagnostic confirmation were excluded, as were those in whom the additional lesion was suspected of being a metastasis of the first. The final sample comprised 11 patients (8 males and 3 females). The previously recognized primary tumors and the suspicion of the new primary site were recorded, as well as their histological classification. Percutaneous procedures guided by CT are safe, well-established techniques. Biopsies guided by functional studies, such as PET/CT, have been widely studied in the literature. For each case, we analyzed the procedure performed by interventional radiology in order to elucidate the diagnosis, as well as other treatments and the outcome. All examinations were performed in a 128-slice multidetector PET/CT scanner (Discovery 610; General Electric, Milwaukee, WI, USA), after a ≥ 6-h fast. The patients received an intravenous solution of 18F-FDG, with an activity count of 3.7-5.2 MBq/kg (0.10-0.14 mCi/kg). The PET/CT images were acquired after 60 to 120 minutes. Post-processing was performed with multiplanar reconstructions and maximum intensity projection techniques. A radiologist with seven years of experience in abdominal diagnostic imaging and a nuclear physician with ten years of experience in PET/CT examinations, together with two radiology residents, analyzed the imaging studies, determining the quantity and location of the lesions. In addition, clinical records, pathology reports, and outcomes were analyzed. Details such as patient age at the time of diagnosis of each tumor, patient gender, tumor type (metachronous or synchronous), tumor site of origin, diagnostic method, histological classification, and treatment regimen were all recorded. All data were tabulated and analyzed in a Microsoft Excel spreadsheet. RESULTS The age of the patients ranged from 52 to 80 years. New primary malignancies were identified in 11 patients, one new tumor being found in ten and two new tumors being found in one. The confirmed sites of the additional malignancies were the lung (n = 4), kidney (n = 3), prostate (n = 2), jejunum (n = 2), and breast (n = 1). In all patients, histology and immunohistochemistry showed that the new lesions were clearly different (i.e., primary) malignancies, rather than metastases of the previously recognized primary lesion. In one patient with a previously recognized neoplasm of the penis, a CT scan showed two new lesions, one a clear cell renal carcinoma and another an adenocarcinoma of the jejunum (Figure 1). Ten patients had single or multiple percutaneous biopsies, guided either by ultrasound or CT, and one patient underwent a surgical procedure for diagnostic and therapeutic purposes. Of the 12 new tumors found, six were synchronous and six were metachronous. Among the suspected or previously recognized malignancies, there were two hepatocellular carcinomas; three adenocarcinomas of the lung (Figure 2), two of which had metastasized to the mediastinum, adrenal glands, and liver (Figure 3); one thymoma; one renal clear cell carcinoma with metastasis to the ipsilateral adrenal gland; one squamous cell carcinoma of the penis; one invasive ductal breast carcinoma (Figure 4); one adenocarcinoma of prostate; and one squamous cell carcinoma of the esophagus (Table 1). 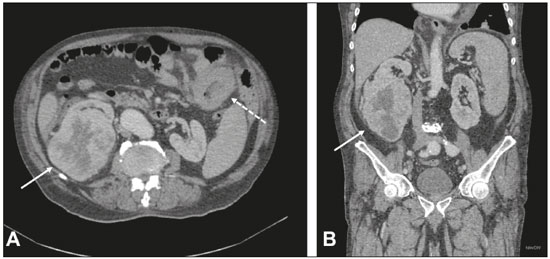 Figure 1. 60-year-old male undergoing restaging of a previously resected squamous cell carcinoma of the penis. Axial and coronal CT (A and B, respectively) showing multiple pulmonary nodules, a mass in the right kidney (arrows), a lesion in the jejunum (dashed arrow), and inguinal lymph node enlargement on the right. A percutaneous biopsy confirmed primary adenocarcinoma of the jejunum with pulmonary and inguinal metastases and clear cell carcinoma in the right kidney. 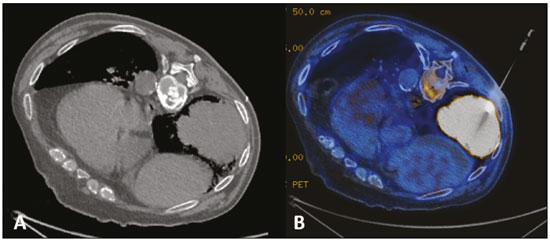 Figure 2. 56-year-old male patient. Axial CT scan showing a voluminous lesion in the lower lobe of the left lung (A). Biopsy guided by 18F-FDG PET/CT showed homogeneous uptake, with no areas of necrosis, increasing the diagnostic accuracy of the procedure and revealing adenocarcinoma (B). 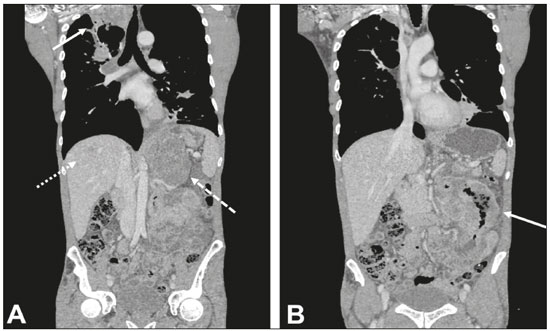 Figure 3. 62-year-old male with recurrent hemoptysis, weight loss, and abdominal pain. A: CT showing a cavitary pulmonary lesion (arrow), a right adrenal mass (dashed arrow), and a hepatic nodule (dotted arrow). B: Asymmetrical circumferential thickening of the jejunum (arrow). Pathology report after percutaneous biopsy confirmed adenocarcinoma of the jejunum and squamous cell carcinoma of the lung with metastases to the adrenal glands and liver. 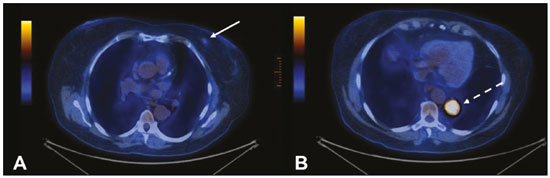 Figure 4. 71-year-old female under investigation for a chronic cough. 18F-FDG PET/CT showed a focal mass in the left breast (arrow in A), confirmed as a spiculated nodule in the ultrasound and a lesion with avid uptake in the right lower lobe of the left lung (dashed arrow in B). Percutaneous biopsy confirmed invasive ductal carcinoma in the left breast and primary adenocarcinoma of the lung. 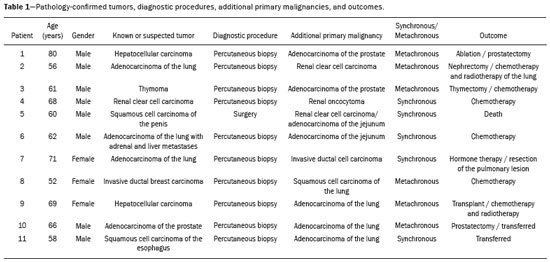 Treatments ranged from chemotherapy alone (in three cases); chemotherapy and surgical resection (in one case); chemotherapy and radiotherapy with surgical resection (in one case); hormone therapy with surgical resection (in one case); ablation of hepatocellular carcinoma and surgical resection (in one case); and transplantation combined with chemotherapy and radiotherapy (in one case). Only one patient progressed to death, three months after the diagnosis of new lesions. Two patients were transferred to other centers for monitoring and treatment, one of them undergoing surgical resection prior to the transfer (Table 1). DISCUSSION Multiple primary tumors can be defined as more than one synchronous or metachronous lesion in the same individual. By definition, multiple primary tumors are histologically different, involving different organs, and metastatic lesions are excluded. Two tumors are categorized as synchronous when they correspond to another tumor site in the same patient and are diagnosed within six months of each other, whereas the second tumor would be categorized as metachronous if diagnosed more than six months after the diagnosis of the index tumor(1). The risk of developing a new primary tumor is 20% higher in patients with an existing neoplasm than in the general population(10). Approximately one third of cancer patients over the age of 60 are diagnosed with an additional primary lesion. The most common risk factors are genetic predisposition, lifestyle, hormonal imbalance, environmental exposures, and previous treatment of a primary tumor(11). Some types of neoplasms tend to be grouped by the risk factors they share: smoking in cancers of the lung, head, and neck; dietary or endocrine factors in gynecological cancers; ultraviolet light in melanoma and skin cancer; and viral agents in cervical and anogenital cancers. Subsequent additional primary malignancies may also be associated with a potentially carcinogenic treatment of the initial lesion, such as chemotherapy, radiotherapy, or both. In addition, genetic risk factors such as BRCA1 and BRCA2 mutations have been linked to a predisposition to multiple malignancies, such as breast cancer and ovarian cancer(12). Genetic conditions can trigger the hereditary cancer syndromes that are characterized by a higher prevalence of neoplasia in individuals of the same family(13) and a high risk of developing tumors at an early age, as well as multiple primary tumors, either metachronous or synchronous(14). Knowledge of these syndromes is important, because the initial diagnosis of an "index" tumor can prompt the investigation of a possible syndromic context and the discovery of other lesions. One example is a diagnosis of hemangioblastoma of the central nervous system, leading to early screening for von Hippel-Lindau syndrome(15); a diagnosis of pulmonary hamartoma, in the context of the Carney triad(16); or a diagnosis of medullary thyroid carcinoma in young patients, in the context of multiple endocrine neoplasia(17). In other, more common, tumors, such as colorectal carcinoma, endometrial carcinoma, and sebaceous neoplasms of the skin, a study of the immunohistochemistry of the lesions can be requested to evaluate any microsatellite instability (DNA mismatch repair) or possible association with Lynch syndrome or Muir-Torre syndrome(18). The detection of unexpected malignant lesions has a significant clinical impact, not only on healthy individuals but also on patients with previously recognized malignant disease. Studies involving patients with previously diagnosed tumors typically focus on the primary disease, and the incidental coexistence of another primary malignant tumor can therefore be missed(19). From a diagnostic point of view, the early recognition and confirmation of such tumors are essential to determining the ideal treatment. Therefore, radiologists should be familiar with the different patterns of presentation in a patient with multiple primary tumors(2). PET with 18F-FDG is being used with increasing frequency in the evaluation and clinical management of an ever greater number of neoplasms(20-23). Some reports also indicate that PET with 18F-FDG has potential as a screening method for cancer and can detect new malignant tumors that other imaging methods fail to detect in asymptomatic individuals(24,25). The PET/CT combination is a promising hybrid imaging modality that is being used routinely in different clinical situations(26-29), because it allows precise integration of metabolic PET images with high-quality CT images in an acquisition that goes from the top of the head to the upper thigh(19). FDG is a glucose analog used as a marker with fluorine-18, a positron-emitting radioisotope, which allows the study of the glucose metabolism because of its higher uptake by tissues with increased glycolysis. A broad spectrum of biochemical changes is present in tumor cells, including higher rates of aerobic and anaerobic glycolysis when compared with those found in normal tissues. The location of 18F-FDG absorption can be precisely determined based on these images, which highlight the metabolic differences between benign and malignant cells(19,21,30). CT-guided biopsy has been widely used as an effective, safe procedure for diagnostic confirmation in various clinical contexts. A biopsy guided by PET/CT, which combines anatomical information obtained from CT and metabolic information from PET with 18F-FDG, is a procedure that can optimize the diagnostic yield of image-guided interventions, given that lesions presenting uptake of 18F-FDG, without a corresponding anatomical anomaly, may be accessible to percutaneous interventions(31). Although there have been no studies demonstrating any significant differences between PET/CT and CT in terms of their ability to obtain a viable biopsy sample or their complication rates, we believe that PET/CT is a particularly important method, given that CT has been shown to fail to detect lesions that later appear as foci of avid 18F-FDG uptake(31). Image-guided percutaneous biopsy is a well-established and safe technique and plays a crucial role in the management of oncology patients. Improvements in the design of needles, the development of new biopsy techniques, and ongoing technological advancements in image orientation have improved the safety and effectiveness of the procedures. Lesions that were previously considered relatively inaccessible can now be accessed safely(32). Some of the advantages of interventional radiology include the possibility of performing complex procedures with small incisions, decreasing the likelihood of infections, promoting the rapid recuperation of the patient, and reducing hospitalization time, given that the techniques involved are minimally invasive, safe, and highly effective(6). CONCLUSION 18F-FDG PET/CT can be used as a tool to complement imaging methods such as CT(30) and, when combined with minimally invasive procedures in interventional radiology, can be useful in the identification of additional primary malignancies the early recognition and diagnosis of which are essential, because the management of cases is often changed if this information is available. Studies show that, although false-positives can occur, the prevalence of true-positives is substantial(19). Additional primary malignancies are often identified in the initial phase, and the chance of a cure is therefore excellent if such malignancies are treated promptly and aggressively(19). Biopsies guided by 18F-FDG PET/CT can help in difficult situations, especially when it is important to know which part of the tumor is active or which lesion is active in patients with multiple, disseminated lesions(33). REFERENCES 1. Shah SA, Riaz U, Zahoor I, et al. Carcinoma multiplex. J Coll Physicians Surg Pak. 2013;23:290-2. 2. Testori A, Cioffi U, De Simone M, et al. Multiple primary synchronous malignant tumors. BMC Res Notes. 2015;8:730. 3. Barber TW, Duong CP, Leong T, et al. 18F-FDG PET/CT has a high impact on patient management and provides powerful prognostic stratification in the primary staging of esophageal cancer: a prospective study with mature survival data. J Nucl Med. 2012;53:864-71. 4. Agress H Jr, Cooper BZ. Detection of clinically unexpected malignant and premalignant tumors with whole-body FDG PET: histopathologic comparison. Radiology. 2004;230:417-22. 5. Hiraoka A, Hirooka M, Ochi H, et al. Importance of screening for synchronous malignant neoplasms in patients with hepatocellular carcinoma: impact of FDG PET/CT. Liver Int. 2013;33:1085-91. 6. O'Brien B, van der Putten W. Quantification of risk-benefit in interventional radiology. Radiat Prot Dosimetry. 2008;129:59-62. 7. Suzuki T, Takahashi H, Yao K, et al. Multiple primary malignancies in the head and neck: a clinical review of 121 patients. Acta Otolaryngol Suppl. 2002;(547):88-92. 8. Morris LGT, Sikora AG, Patel SG, et al. Second primary cancers after an index head and neck cancer: subsite-specific trends in the era of human papillomavirus-associated oropharyngeal cancer. J Clin Oncol. 2011;29:739-46. 9. Cheng HY, Chu CH, Chang WH, et al. Clinical analysis of multiple primary malignancies in the digestive system: a hospital-based study. World J Gastroenterol. 2005;11:4215-9. 10. Luciani A, Balducci L. Multiple primary malignancies. Semin Oncol. 2004;31:264-73. 11. Soerjomataram I, Coebergh JW. Epidemiology of multiple primary cancers. Methods Mol Biol. 2009;471:85-105. 12. Wooster R, Weber BL. Breast and ovarian cancer. N Engl J Med. 2003;348: 2339-47. 13. Dantas ELR, Lima Sá FH, Carvalho SMF, et al. Genética do câncer hereditário. Rev Bras Cancerol. 2009;55:263-9. 14. Rahner N, Steinke V. Hereditary cancer syndromes. Dtsch Arztebl Int. 2008;105:706-14. 15. Ortiz-Velázquez RI, Santos-Franco JA, Caldas JGMP, et al. Hemangioblastomas aparentemente esporádicos e doença de von Hippel-Lindau não suspeita. Arq Bras Neurocir. 2008;27:67-73. 16. Fiala L, Kocáková I, Simunek R, et al. Carney triad. Rozhl Chir. 2017;96:267-72. 17. Maia AL, Siqueira DR, Kulcsar MA, et al. Diagnosis, treatment, and follow-up of medullary thyroid carcinoma: recommendations by the Thyroid Department of the Brazilian Society of Endocrinology and Metabolism. Arq Bras Endocrinol Metabol. 2014;58:667-700. 18. Kacerovská D, Kazakov DV, Cerná K, et al. Muir-Torre syndrome-a phenotypic variant of Lynch syndrome. Cesk Patol. 2010;46:86-94. 19. Ishimori T, Patel PV, Wahl RL. Detection of unexpected additional primary malignancies with PET/CT. J Nucl Med. 2005;46:752-7. 20. Coleman RE. PET in lung cancer. J Nucl Med. 1999;40:814-20. 21. Delbeke D. Oncological applications of FDG PET imaging: brain tumors, colorectal cancer, lymphoma and melanoma. J Nucl Med. 1999;40:591-603. 22. Delbeke D. Oncological applications of FDG PET imaging. J Nucl Med. 1999;40:1706-15. 23. Gambhir SS, Czernin J, Schwimmer J, et al. A tabulated summary of the FDG PET literature. J Nucl Med. 2001;42(5 Suppl):1S-93S. 24. Yasuda S, Ide M, Fujii H, et al. Application of positron emission tomography imaging to cancer screening. Br J Cancer. 2000;83:1607-11. 25. Shen YY, Su CT, Chen GJ, et al. The value of 18F-fluorodeoxyglucose positron emission tomography with the additional help of tumor markers in cancer screening. Neoplasma. 2003;50:217-21. 26. Meirelles GSP, Capobianco J, Oliveira MAC. Pitfalls and artifacts in the interpretation of oncologic PET/CT of the chest. Radiol Bras. 2017;50:55-9. 27. Farias LPG, Padilha IG, Lemos MLR, et al. Pulmonary cryptococcosis mimicking neoplasm in terms of uptake PET/CT. Radiol Bras. 2018;51:63-4. 28. Prado Júnior LM, Marino FM, Barra R, et al. One-year experience with 68Ga-PSMA PET/CT: applications and results in biochemical recurrence of prostate cancer. Radiol Bras. 2018;51:151-5. 29. Bitencourt AGV, Andrade WP, Cunha RR, et al. Detection of distant metastases in patients with locally advanced breast cancer: role of 18F-fluorodeoxyglucose positron emission tomography/computed tomography and conventional imaging with computed tomography scans. Radiol Bras. 2017;50:211-5. 30. Soares Junior J, Fonseca RP, Cerci JJ, et al. Recommendations on the use of 18F-FDG PET/CT in Oncology. Consensus between the Brazilian Society of Cancerology and the Brazilian Society of Biology, Nuclear Medicine and Molecular Imaging. Radiol Bras. 2010;43:255-9. 31. Cerci JJ, Tabacchi E, Bogoni M, et al. Comparison of CT and PET/CT for biopsy guidance in oncological patients. Eur J Nucl Med Mol Imaging. 2017;44:1269-74. 32. Gupta S, Madoff DC. Image-guided percutaneous needle biopsy in cancer diagnosis and staging. Tech Vasc Interv Radiol. 2007;10:88-101. 33. Govindarajan MJ, Nagaraj KR, Kallur KG, et al. PET/CT guidance for percutaneous fine needle aspiration cytology/biopsy. Indian J Radiol Imaging. 2009;19:208-9. 1. Hospital Universitário Maria Aparecida Pedrossian da Universidade Federal de Mato Grosso do Sul (HUMAP-UFMS), Campo Grande, MS, Brazil; https://orcid.org/0000-0001-5930-1383 2. Hospital Universitário Maria Aparecida Pedrossian da Universidade Federal de Mato Grosso do Sul (HUMAP-UFMS), Campo Grande, MS, Brazil; https://orcid.org/0000-0002-8679-7369 3. MS Diagnósticos Médicos, Campo Grande, MS, Brazil; https://orcid.org/0000-0002-4799-2700 4. Universidade Federal de Minas Gerais (UFMG), Belo Horizonte, MG, Brazil; https://orcid.org/0000-0002-4258-2198 5. MS Diagnósticos Médicos, Campo Grande, MS, Brazil; https://orcid.org/0000-0002-5475-8787 6. Instituto de Tratamento do Câncer (ITC), Campo Grande, MS, Brazil; https://orcid.org/0000-0002-5948-2185 7. Universidade Federal do Rio de Janeiro (UFRJ), Rio de Janeiro, RJ, Brazil; https://orcid.org/0000-0001-8797-7380 8. Hospital Universitário Maria Aparecida Pedrossian da Universidade Federal de Mato Grosso do Sul (HUMAP-UFMS), Campo Grande, MS, Brazil; https://orcid.org/0000-0003-0006-3725 Correspondence: Dr Thiago Franchi Nunes Avenida Senador Filinto Müller, 355, Vila Ipiranga Campo Grande, MS, Brazil, 79080-190 Email: thiagofranchinunes@gmail.com Received February 14, 2018 Accepted after revision May 25, 2018 |
|
Av. Paulista, 37 - 7° andar - Conj. 71 - CEP 01311-902 - São Paulo - SP - Brazil - Phone: (11) 3372-4544 - Fax: (11) 3372-4554