Radiologia Brasileira - Publicação Científica Oficial do Colégio Brasileiro de Radiologia
AMB - Associação Médica Brasileira CNA - Comissão Nacional de Acreditação
 Vol. 51 nº 2 - Mar. / Apr. of 2018
Vol. 51 nº 2 - Mar. / Apr. of 2018
|
ORIGINAL ARTICLE
|
|
Bone marrow uptake of 18F-fluorodeoxyglucose in Hodgkin lymphoma without bone involvement: comparison between patients with and without B symptoms |
|
|
Autho(rs): Rômulo Hermeto Bueno do Vale1; Daniela Andrade Ferraro1; Paulo Schiavom Duarte2; Giovana Carvalho1; Marcos Santos Lima1; George Barbério Coura Filho2; Marcelo Tatit Sapienza2; Carlos Alberto Buchpiguel2 |
|
|
Keywords: Keywords: Fluorodeoxyglucose F18; Positron emission tomography computed tomography/methods; Hodgkin disease; Bone marrow/diagnostic imaging. |
|
|
Abstract: INTRODUCTION
Hodgkin lymphoma accounts for approximately 12% of all cases of lymphoma and 1% of all malignancies(1). The therapies used in the initial treatment depend on the stage of the disease at diagnosis(2–4). Therefore, appropriate staging before the initiation of therapy is crucial(5). Lymphoma staging, which is based on the Ann Arbor system(6,7), usually involves computed tomography and bone marrow biopsy. Functional imaging employing 18F-fluorodeoxyglucose positron emission tomography/computed tomography (18F-FDG PET/CT) has come into widespread use in the management of Hodgkin lymphoma. Because 18F-FDG PET/CT is accurate at differentiating residual viable tumor from therapy-induced fibrosis(8), it has been incorporated into the recently revised criteria for end-of-therapy assessment(9). In addition, various studies have suggested that 18F-FDG PET/CT can assess the treatment response early in the course of therapy for Hodgkin lymphoma, thus allowing the therapy to be tailored to each patient, on the basis of the individual risk of relapse(10,11). Data from a baseline examination increases the accuracy of the 18F-FDG PET/CT assessment of the treatment response Performing 18F-FDG PET/CT at baseline has therefore been strongly encouraged in cases of Hodgkin lymphoma. A pre-treatment 18F-FDG PET/CT scan can also provide information that is useful for the initial staging(12) and for the implementation of radiotherapy(13). Diffuse uptake of 18F-FDG in the axial skeleton has been described in cases of diffuse bone marrow infiltration of malignant lymphoma(14) and diffuse bone marrow metastases(15). Benign diffuse bone marrow 18F-FDG uptake secondary to bone marrow stimulation by granulocyte-macrophage stimulating factor(16), granulocyte colony-stimulating factor, or erythropoietin(17) has also been reported. There have also been reports of diffuse bone marrow 18F-FDG uptake resulting from hematologic diseases, including chronic myeloid leukemia(18) and myelofibrosis(19). However, we have noted diffuse bone marrow uptake in some patients before the onset of treatment, without bone marrow infiltration and without the use of granulocyte colony-stimulating factor or erythropoietin. We hypothesized that this uptake would be associated with the presence of inflammatory (B) symptoms. The objective of this study was to compare the degree of diffuse benign bone marrow uptake of 18F-FDG in Hodgkin lymphoma patients with and without B symptoms. MATERIALS AND METHODS Patient population We reviewed the medical charts of 74 Hodgkin lymphoma patients who underwent 18F-FDG PET/CT studies prior to the initiation of therapy, between October 2010 and September 2013. All patients had a negative bone marrow biopsy and 18F-FDG PET/CT images that were not suggestive of bone marrow involvement. Therefore, given that both methods are complementary for the diagnosis of bone marrow involvement(20), the patients were considered free of bone marrow disease. The following 18F-FDG PET/CT patterns were considered suggestive of bone marrow infiltration: focal, multifocal, or heterogeneous bone marrow uptake; and any suspicious alterations on the CT scan. Patients presenting any pattern suggestive of bone marrow infiltration were excluded from the analysis. One patient with a femoral prosthesis was also excluded because the prosthesis produced an artifact in the images, impairing the local analysis. Of the 74 patients evaluated, 54 (73%) presented B symptoms. Hodgkin lymphoma was diagnosed by histopathology and immunophenotyping. The disease stage was determined clinically according to the Ann Arbor system. The presence of B symptoms was defined as fever > 38ºC, night sweats, and weight loss > 10% over a period of ≤ six months, as determined by reviewing patient charts and based on the classifications established by the referring physician. On the basis of the histopathological analysis of the lymphoma, some of the patients were categorized as having classic Hodgkin lymphoma. The remaining patients were stratified by pathologic lymphoma subtype: nodular sclerosis; mixed cellularity; lymphocyte-predominant; or lymphocyte-depleted. Lymphomas were staged according to the Ann Arbor classification, and patients were characterized by age and gender. Image acquisition Each patient underwent a three-dimensional PET/CT scan from skull base to mid-thigh approximately 60 min after injection of 370 MBq (10 mCi) of 18F-FDG. Images were obtained on a PET/CT scanner with time-of-flight technology (Discovery PET/CT 690; GE Healthcare, Milwaukee, WI, USA). The PET images were acquired for 3 min per bed position (15-cm slice thickness with a 3-cm overlap). The iterative technique with 24 subsets was used for PET image reconstruction in all studies. For attenuation correction and diagnostic purposes, we obtained non-contrast-enhanced CT transmission scans using the following parameters: current, 125 mAs; voltage, 120 kVp; gantry rotation, 0.5 s; pitch, 1.375; and axial slice thickness, 3.75 mm. Image analysis As illustrated in Figure 1, elliptical regions of interest (ROIs), each measuring 2.5–3.0 cm at its greatest diameter, were drawn on the sternum, the proximal thirds of the humeri, the proximal thirds of the femora, and both iliac wings (totaling seven ROIs per patient). For all patients, the ROIs were drawn by the same nuclear physician, who was blinded to the symptom group. 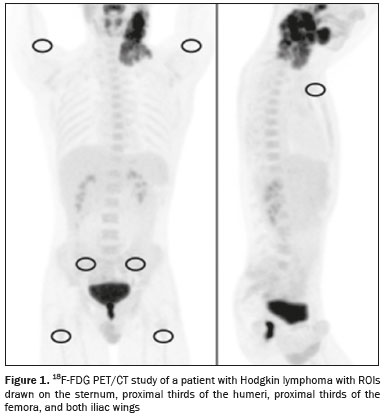 Figure 1. 18F-FDG PET/CT study of a patient with Hodgkin lymphoma with ROIs drawn on the sternum, proximal thirds of the humeri, proximal thirds of the femora, and both iliac wings Statistical analysis For each of the seven ROIs, the groups with and without B symptoms were compared, in terms of the maximum standardized uptake value (SUVmax), with the Mann-Whitney U test. We also compared the two groups, in terms of the pathologic subtype, disease stage, patient age, and patient gender, using the Mann-Whitney U test (for patient age) or the chi-square test (for the remaining variables). RESULTS The characteristics of the two groups of patients are presented in Table 1. As can be seen in the table, there was no statistically significant difference between the two groups in terms of age. We observed a predominance of the nodular sclerosis subtype in the B symptoms group. In addition, there was a tendency toward more advanced stages of lymphoma in the B symptoms group, with borderline significance (p = 0.12). 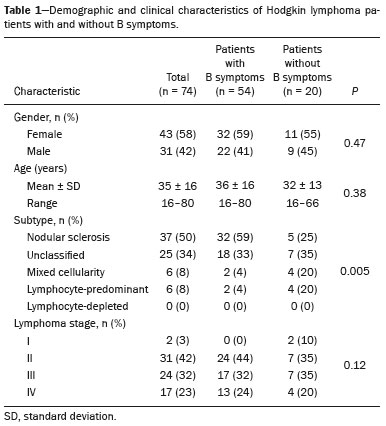 The mean, standard deviation, and range of the SUVmax for each of the seven ROIs are presented, by group, in Table 2, as are the corresponding p-values. For six of the seven ROIs, there were statistically significant differences between the two groups in terms of the SUVmax, which was higher in the B symptoms group. There was also a tendency toward significantly higher SUVmax for the right iliac wing ROIs in the B symptoms group (p = 0.06). Examples of 18F-FDG PET/CT studies of patients with and without B symptoms are shown in Figure 2. 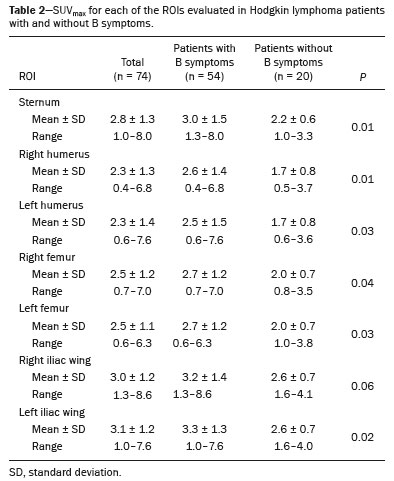 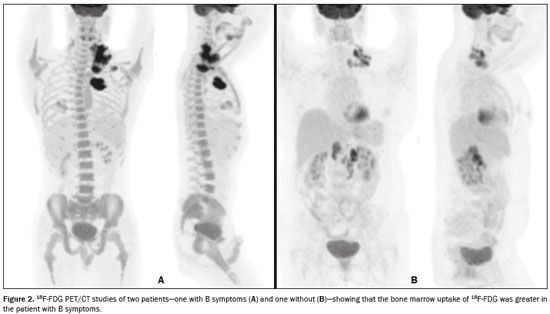 Figure 2. 18F-FDG PET/CT studies of two patients—one with B symptoms (A) and one without (B)—showing that the bone marrow uptake of 18F-FDG was greater in the patient with B symptoms. DISCUSSION Recent studies conducted in Brazil have highlighted the importance of functional imaging with single-photon emission CT and PET/CT using 18F-FDG to improving the diagnosis of several diseases(21–26). In the present cross-sectional study, we have demonstrated an association between the presence of B symptoms and greater benign bone marrow uptake of 18F-FDG in patients with Hodgkin lymphoma. Although the reasons for this association are unknown, it could be related to the production of cytokines in the tumor microenvironment. Neoplastic Hodgkin and Reed-Sternberg cells interact with reactive cells of the tumor microenvironment, and that interaction has been reported to be associated with high levels of cytokines(27). In addition, the local production of those cytokines results in elevated systemic levels in the peripheral blood, which leads to the development of systemic symptoms and biochemical abnormalities that are correlated with disease prognosis(28). It has also reported that interleukin (IL)-6 is the cytokine most closely associated with lymphopenia and B symptoms in lymphoma patients(29), as well as that serum IL-6 levels are higher in Hodgkin lymphoma patients with B symptoms(30). The levels of hepcidin, the expression of which is induced by IL-6, have been shown to be higher in patients with more aggressive disease characteristics, such as stage IV disease, B symptoms, and an International Prognostic Score > 2(31). The levels of IL-9 have also been shown to correlate with B symptoms(32). However, there have also been studies showing that stimulation of hematopoietic cytokines can cause a diffuse increase in bone marrow accumulation of 18F-FDG, mimicking bone marrow metastasis, on PET imaging(33,34). In one study, conducted by Salaun et al.(35), the degree of diffuse bone marrow uptake at the initial staging of Hodgkin lymphoma was correlated with the level of C-reactive protein, an inflammatory marker. The authors concluded that, although diffuse bone marrow uptake at the initial staging of Hodgkin lymphoma could be due to bone marrow involvement, it was more likely due to inflammatory changes in the bone marrow. Therefore, in the neoplastic cell microenvironment, the increased diffuse bone marrow uptake of 18F-FDG in Hodgkin's lymphoma patients with B symptoms could be mediated by the increased production of cytokines, which are inflammatory modulators. It is noteworthy that those authors also found a statistically significant association between the SUVmax in the sacrum and the presence of B symptoms. Therefore, their findings support the results of our study, in which we analyzed a large number of bone regions. Our study has certain limitations. The statistically significant differences between our groups of patients with and without B symptoms, in terms of the lymphoma subtypes, have the potential to confound the results. The predominance of the nodular sclerosis subtype in the B symptoms group is not an unexpected finding, a previous study having shown a difference between patients with and without B symptoms in terms of the prevalence of Hodgkin lymphoma subtypes(36). However, that difference does not alter our conclusions, because our hypothesis was that there would be an association, rather than a causal relationship, between B symptoms and increased 18F-FDG uptake in bone marrow. Another potential limitation of our study is related to the accuracy of the information regarding B symptoms. Information about B symptoms was obtained from patient charts, based on the reporting of the referring physicians, and might therefore be inaccurate. However, there is no reason for such inaccuracies to occur in one particular direction (favoring the presence or absence of symptoms) and they tend to diminish the strength of an association rather than increasing it. The fact this was a cross-sectional study could also be seen as a limitation, because cross-sectional analyses use data collected for other purposes and are often unable to include all data on confounding variables that potentially affect the relationship between cause and effect. Nevertheless, as previously mentioned, we did not hypothesize a causal relationship between B symptoms and benign 18F-FDG bone marrow uptake. Therefore, because we believe that B symptoms and benign bone marrow uptake of 18F-FDG could both be attributed to upregulation of cytokine production, a cross-sectional analysis seems well suited to testing our hypothesis. CONCLUSION In our sample of patients with Hodgkin lymphoma, the presence of B symptoms was associated with a benign diffuse increase in the uptake of 18F-FDG in bone marrow. REFERENCES 1. Diehl V. Hodgkin's disease—from pathology specimen to cure. N Engl J Med. 2007;357:1968–71. 2. Connors JM. State-of-the-art therapeutics: Hodgkin's lymphoma. J Clin Oncol. 2005;23:6400–8. 3. Diehl V, Fuchs M. Early, intermediate and advanced Hodgkin's lymphoma: modern treatment strategies. Ann Oncol. 2007;18 Suppl 9:ix71–9. 4. Eghbali H, Raemaekers J, Carde P. The EORTC strategy in the treatment of Hodgkin's lymphoma. Eur J Haematol Suppl. 2005;(66):135–40. 5. Favier O, Heutte N, Stamatoullas-Bastard A, et al. Survival after Hodgkin lymphoma: causes of death and excess mortality in patients treated in 8 consecutive trials. Cancer. 2009;115:1680–91. 6. Lister TA, Crowther D, Sutcliffe SB, et al. Report of a committee convened to discuss the evaluation and staging of patients with Hodgkin's disease: Cotswolds meeting. J Clin Oncol. 1989;7:1630–6. 7. Carbone PP, Kaplan HS, Musshoff K, et al. Report of the Committee on Hodgkin's Disease Staging Classification. Cancer Res. 1971;31:1860–1. 8. Weihrauch MR, Re D, Scheidhauer K, et al. Thoracic positron emission tomography using 18F-fluorodeoxyglucose for the evaluation of residual mediastinal Hodgkin disease. Blood. 2001;98:2930–4. 9. Barrington SF, Mikhaeel NG, Kostakoglu L, et al. Role of imaging in the staging and response assessment of lymphoma: consensus of the International Conference on Malignant Lymphomas Imaging Working Group. J Clin Oncol. 2014;32:3048–58. 10. Furth C, Steffen IG, Amthauer H, et al. Early and late therapy response assessment with [18F]fluorodeoxyglucose positron emission tomography in pediatric Hodgkin's lymphoma: analysis of a prospective multicenter trial. J Clin Oncol. 2009;27:4385–91. 11. Gallamini A, Hutchings M, Rigacci L, et al. Early interim 2-[18F]fluoro-2-deoxy-D-glucose positron emission tomography is prognostically superior to international prognostic score in advanced-stage Hodgkin's lymphoma: a report from a joint Italian-Danish study. J Clin Oncol. 2007;25:3746–52. 12. Hutchings M, Loft A, Hansen M, et al. Position emission tomography with or without computed tomography in the primary staging of Hodgkin's lymphoma. Haematologica. 2006;91:482–9. 13. Girinsky T, Specht L, Ghalibafian M, et al. The conundrum of Hodgkin lymphoma nodes: to be or not to be included in the involved node radiation fields. The EORTC-GELA lymphoma group guidelines. Radiother Oncol. 2008;88:202–10. 14. Carr R, Barrington SF, Madan B, et al. Detection of lymphoma in bone marrow by whole-body positron emission tomography. Blood. 1998;91:3340–6. 15. Lewanski CR, Kaplan GR, Potter J, et al. Bone marrow involvement in breast cancer detected by positron emission tomography. J R Soc Med. 1999;92:193–5. 16. Kazama T, Swanston N, Podoloff DA, et al. Effect of colony-stimulating factor and conventional- or high-dose chemotherapy on FDG uptake in bone marrow. Eur J Nucl Med Mol Imaging. 2005;32:1406–11. 17. Blodgett TM, Ames JT, Torok FS, et al. Diffuse bone marrow uptake on whole-body F-18 fluorodeoxyglucose positron emission tomography in a patient taking recombinant erythropoietin. Clin Nucl Med. 2004;29:161–3. 18. Takalkar A, Yu JQ, Kumar R, et al. Diffuse bone marrow accumulation of FDG in a patient with chronic myeloid leukemia mimics hematopoietic cytokine-mediated FDG uptake on positron emission tomography. Clin Nucl Med. 2004;29:637–9. 19. Burrell SC, Fischman AJ. Myelofibrosis on F-18 FDG PET imaging. Clin Nucl Med. 2005;30:674. 20. Weiler-Sagie M, Kagna O, Dann EJ, et al. Characterizing bone marrow involvement in Hodgkin's lymphoma by FDG-PET/CT. Eur J Nucl Med Mol Imaging. 2014;41:1133–40. 21. Mosmann MP, Borba MA, Macedo FPN, et al. Solitary pulmonary nodule and 18F-FDG PET/CT. Part 1: epidemiology, morphological evaluation and cancer probability. Radiol Bras. 2016;49:35–42. 22. Mosmann MP, Borba MA, Macedo FPN, et al. Solitary pulmonary nodule and 18F-FDG PET/CT. Part 2: accuracy, cost-effectiveness, and current recommendations. Radiol Bras. 2016;49:104–11. 23. Sabino D, Vale RHB, Duarte PS, et al. Complementary findings on 18F-FDG PET/CT and 18F-NaF PET/CT in a patient with Erdheim-Chester disease. Radiol Bras. 2017;50:202–3. 24. Bitencourt AGV, Andrade WP, Cunha RR, et al. Detection of distant metastases in patients with locally advanced breast cancer: role of 18F-fluorodeoxyglucose positron emission tomography/computed tomography and conventional imaging with computed tomography scans. Radiol Bras. 2017;50:211–5. 25. Monteiro PHS, Souza TF, Moretti ML, et al. SPECT/CT with radiolabeled somatostatin analogues in the evaluation of systemic granulomatous infections. Radiol Bras. 2017;50:378–82. 26. Vale RHB, Sado HN, Danilovic DLS, et al. Incidental diagnosis of struma ovarii through radioiodine whole-body scanning: incremental role of SPECT/CT. Radiol Bras. 2016;49:126–7. 27. Skinnider BF, Mak TW. The role of cytokines in classical Hodgkin lymphoma. Blood. 2002;99:4283–97. 28. Casasnovas RO, Mounier N, Brice P, et al. Plasma cytokine and soluble receptor signature predicts outcome of patients with classical Hodgkin's lymphoma: a study from the Groupe d'Etude des Lymphomes de l'Adulte. J Clin Oncol. 2007;25:1732–40. 29. Uskudar Teke H, Gulbas Z, Bal C. Serum levels of cytokines and prevalence of autoantibodies in lymphoma patients and their prognostic value. J BUON. 2014;19:191–7. 30. Gaiolla RD, Domingues MA, Niéro-Melo L, et al. Serum levels of interleukins 6, 10, and 13 before and after treatment of classic Hodgkin lymphoma. Arch Pathol Lab Med. 2011;135:483–9. 31. Hohaus S, Massini G, Giachelia M, et al. Anemia in Hodgkin's lymphoma: the role of interleukin-6 and hepcidin. J Clin Oncol. 2010;28:2538–43. 32. Fischer M, Bijman M, Molin D, et al. Increased serum levels of interleukin-9 correlate to negative prognostic factors in Hodgkin's lymphoma. Leukemia. 2003;17:2513–6. 33. Inoue K, Okada K, Harigae H, et al. Diffuse bone marrow uptake on F-18 FDG PET in patients with myelodysplastic syndromes. Clin Nucl Med. 2006;31:721–3. 34. Moulin-Romsee G, Hindié E, Cuenca X, et al. (18)F-FDG PET/CT bone/bone marrow findings in Hodgkin's lymphoma may circumvent the use of bone marrow trephine biopsy at diagnosis staging. Eur J Nucl Med Mol Imaging. 2010;37:1095–105. 35. Salaun PY, Gastinne T, Bodet-Milin C, et al. Analysis of 18F-FDG PET diffuse bone marrow uptake and splenic uptake in staging of Hodgkin's lymphoma: a reflection of disease infiltration or just inflammation? Eur J Nucl Med Mol Imaging. 2009;36:1813–21. 36. Shimabukuro-Vornhagen A, Haverkamp H, Engert A, et al. Lymphocyte-rich classical Hodgkin's lymphoma: clinical presentation and treatment outcome in 100 patients treated within German Hodgkin's Study Group trials. J Clin Oncol. 2005;23:5739–45. 1. MD, Division of Nuclear Medicine, Instituto do Câncer do Estado de São Paulo (Icesp), São Paulo, SP, Brazil 2. MD, PhD, Division of Nuclear Medicine, Instituto do Câncer do Estado de São Paulo (Icesp), São Paulo, SP, Brazil Study conducted at the Instituto do Câncer do Estado de São Paulo (Icesp), São Paulo, SP, Brazil. Mailing address: Dra. Daniela Andrade Ferraro Instituto do Câncer do Estado de São Paulo – Medicina Nuclear Avenida Doutor Arnaldo, 251, 4-SS, Consolação São Paulo, SP, Brazil, 01246-000 E-mail: daniela.ferraro@hc.fm.usp.br Received November 7, 2016. Accepted after revision March 20, 2017. |
|
Av. Paulista, 37 - 7° andar - Conj. 71 - CEP 01311-902 - São Paulo - SP - Brazil - Phone: (11) 3372-4544 - Fax: (11) 3372-4554