Radiologia Brasileira - Publicação Científica Oficial do Colégio Brasileiro de Radiologia
AMB - Associação Médica Brasileira CNA - Comissão Nacional de Acreditação
 Vol. 50 nº 1 - Jan. /Feb. of 2017
Vol. 50 nº 1 - Jan. /Feb. of 2017
|
LETTER TO THE EDITOR
|
|
Dural fistula with bilateral arterial supply, mimicking a brainstem tumor |
|
|
Autho(rs): Bárbara Liaffa; Fábio Noro; Paulo Roberto Valle Bahia; Flávia Pinto Dezonne Motta; Edson Marchiori |
|
|
Dear Editor,
A 73-year-old woman presented with a history of at least four episodes of deep vein thrombosis. In the last five months, she had experienced severe ataxia, difficulty in swallowing, bilateral tinnitus, and symptoms related to intracranial hypertension, such as nausea and vomiting. Magnetic resonance imaging (MRI) revealed a hyperintense signal on T2-weighted images and an enlarged brainstem, the swelling extending to the thalamus, cerebellar peduncles, and to the cervical portion of the spinal cord (Figures 1A and 1B). The images could erroneously indicate a diagnosis of brainstem tumor, glioma in particular, due to the infiltrative pattern of the lesion and the increased organ volume. However, thorough evaluation with advanced imaging techniques, such as magnetic susceptibility-weighted sequences, demonstrated an extensive network of dilated peripheral veins, together with pronounced collateral circulation. Cerebral angiography showed a dural arteriovenous fistula (DAVF) with bilateral arterial supply via branches of the maxillary arteries. Venous drainage was mostly through the rectum and galenic system (Figures 1C and 1D). Involvement of the brainstem and cervical spinal cord was due to venous congestive injury. The classical surgical approach was precluded by the deep, inaccessible location, whereas endovascular therapy was precluded by the extensive involvement and bilateral nature of the fistula-sustaining arterial supply. The patient underwent gastrostomy and was discharged to palliative home care. 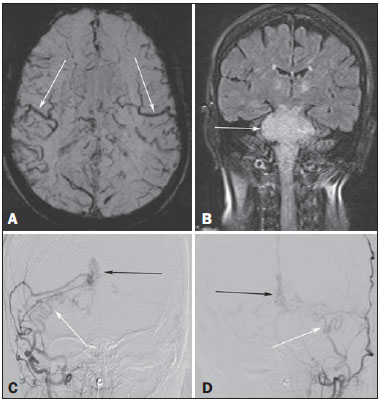 Figure 1. A: Axial slice in a susceptibility-weighted sequence showing numerous large-caliber superficial veins, representing venous congestion. B: Coronal slice in a fluid-attenuated inversion recovery sequence showing a hyperintense signal and increased brainstem volume, mimicking a brain tumor. C,D: Digital angiography with subtraction technique, revealing the bilateral nature of the arterial supply (white arrows) and the nidus (black arrows) formed by the fistula. Vascular lesions are often difficult to diagnose(1–8). DAVFs, which are characterized by abnormal communication between the arterial and venous systems, without intervening capillary beds, account for less than 10% of all cerebral vascular malformations(9). The most common place of occurrence is the transverse sinus(9), and there have been no reports of bilateral arterial supply. The two principal forms of presentation are hemorrhagic and non-hemorrhagic, both typically occurring as a consequence of intracranial venous hypertension(9,10), which appears as the best predictor of poor prognosis(11). Cerebral angiography continues to be the gold standard for the diagnosis of DAVF, in which the nidus represents the arteriovenous shunt itself and collateral vessels develop in order to drain the venous congestion(12). Injury due to venous congestion is an example of a severe non-hemorrhagic manifestation, which can be prevented through the early diagnosis of DAVF, the treatment of choice being endovascular therapy, with the objective of interrupting the arterial supply to the venous system(9). REFERENCES 1. Cardarelli-Leite L, Velloni FG, Salvadori PS, et al. Abdominal vascular syndromes: characteristic imaging findings. Radiol Bras. 2016;49:257–63. 2. Batista MN, Barreto MM, Cavaguti RF, et al. Pulmonary artery sarcoma mimicking chronic pulmonary thromboembolism. Radiol Bras. 2015; 48:333–4. 3. Dias DA, Afonso LHC, Abud DG. Femoral artery injury during aneurysm coiling. Radiol Bras. 2015;48:335–6. 4. Neves PO, Andrade J, Monção H. Coronary anomalies: what the radiologist should know. Radiol Bras. 2015;48:233–41. 5. Amaral RH, Souza VVS, Nin CS, et al. Aortic lesion simulating pulmonary disease: a case report. Radiol Bras. 2014;47:320–2. 6. Ribeiro BNF, Ribeiro RN, Zanetti G, et al. Hughes-Stovin syndrome: an unusual cause of pulmonary artery aneurysms. Radiol Bras. 2016;49: 202–3. 7. Abud TG, Nguyen AD, Abud LG, et al. Anterior cerebral artery aneurysm rupture presenting as hemorrhage in the splenium of the corpus callosum. Radiol Bras. 2016;49:268–9. 8. Abreu Junior L, Kuniyoshi CH, Wolosker AB, et al. Vascular loops in the anterior inferior cerebellar artery, as identified by magnetic resonance imaging, and their relationship with otologic symptoms. Radiol Bras. 2016;49:300–4. 9. Hacein-Bey L, Konstas AA, Pile-Spellman J. Natural history, current concepts, classification, factors impacting endovascular therapy, and pathophysiology of cerebral and spinal dural arteriovenous fistulas. Clin Neurol Neurosurg. 2014;121:64–75. 10. Cognard C, Gobin YP, Pierot L, et al. Cerebral dural arteriovenous fistulas: clinical and angiographic correlation with a revised classification of venous drainage. Radiology. 1995;194:671–80. 11. Cognard C, Casasco A, Toevi M, et al. Dural arteriovenous fistulas as a cause of intracranial hypertension due to impairment of cranial venous outflow. J Neurol Neurosurg Psychiatry. 1998;65:308–16. 12. Signorelli F, Gory B, Maduri R, et al. Intracranial dural arteriovenous fistulas: a review of current management based on emerging knowledge. J Neurosurg Sci. 2015 Feb 13. [Epub ahead of print]. Universidade Federal do Rio de Janeiro (UFRJ), Rio de Janeiro, RJ, Brasil Endereço para correspondência: Dr. Edson Marchiori Rua Thomaz Cameron, 438, Valparaiso Petrópolis, RJ, Brasil, 25685-120 E-mail: edmarchiori@gmail.com |
|
GN1© Copyright 2025 - All rights reserved to Colégio Brasileiro de Radiologia e Diagnóstico por Imagem
Av. Paulista, 37 - 7° andar - Conj. 71 - CEP 01311-902 - São Paulo - SP - Brazil - Phone: (11) 3372-4544 - Fax: (11) 3372-4554
Av. Paulista, 37 - 7° andar - Conj. 71 - CEP 01311-902 - São Paulo - SP - Brazil - Phone: (11) 3372-4544 - Fax: (11) 3372-4554