Radiologia Brasileira - Publicação Científica Oficial do Colégio Brasileiro de Radiologia
AMB - Associação Médica Brasileira CNA - Comissão Nacional de Acreditação
 Vol. 49 nº 2 - Mar. / Apr. of 2016
Vol. 49 nº 2 - Mar. / Apr. of 2016
|
ORIGINAL ARTICLE
|
|
Balloon-based adjuvant radiotherapy in breast cancer: comparison between 99mTc and HDR 192Ir |
|
|
Autho(rs): Tarcísio Passos Ribeiro de Campos1; Carla Flavia de Lima2; Ethel Mizrahy Cuperschmid3 |
|
|
Keywords: Breast brachytherapy; Balloon; Radiotherapy dosage; Radiotherapy, adjuvant; 99mTc; 192Ir; Monte Carlo method. |
|
|
Abstract: INTRODUCTION
Worldwide, breast cancer caused 521,000 deaths in 2012(1). In Brazil, the incidence of breast cancer has risen significantly in the last ten years, increasing the number of deaths. The disease is a consequence of genetic factors in combination with social factors, environmental factors, and life habits(2). The factors that favor carcinogenesis and can cause breast cancer include the following(3,4): exposure to carcinogens, such as chemical products and ionizing radiation; parasitic and viral infections; inherited genetic mutations; hormonal and metabolic changes; and immunodeficiency. Treatment involves surgery, locoregional radiotherapy, chemotherapy, and hormone therapy for systemic control(5,6). Surgical resection margins are defined as a broad margin of possible cancer cell infiltration. The surgical procedure can be conservative or radical(7). Non-conservative surgery involves subcutaneous glandular mastectomy or subcutaneous mastectomy, preserving only the skin and the nipple-areola complex. In contrast, simple or total mastectomy involves removal of the breast including the skin and nipple-areola complex. Modified radical surgery involves mastectomy with partial preservation of the chest muscles and axillary lymphadenectomy, whereas radical surgery involves the complete removal of the chest muscles with axillary lymphadenectomy. Breast conserving surgery includes excision of the tumor with no margin (lumpectomy) or with a margin (segmentectomy or segmental resection). Tumors smaller than 2.0 cm with tumor-free surgical margins can be treated with segmental resection followed by complementary radiotherapy. However, for tumors smaller than 4 cm with tumor-free resection margins, up to 10% of cases treated with conservative treatment involving radiotherapy have been reported to show local recurrence(8-10), leading to psychological trauma and having negative repercussions for the cancer prognosis. Local recurrence depends on the level of aggressiveness and diameter of the tumor, as well as on microscopic involvement of the margins. In breast conserving surgery, complementary (boost) radiotherapy in the tumor area is recommended(8,11,12). Regardless of the histological type of the tumor, age of the patient, chemotherapy, or hormone therapy, and even with surgical resection margins free of cancer cells, breast conserving surgery is followed by breast irradiation(7). A radiotherapy boost in the area is indicated when patients are below 50 years of age, specimens show more than 25% ductal carcinoma in situ, exiguous margins are less than 1 cm (whether free of cancer cells or not), and the tumor shows a high degree of local aggressiveness(8-10). Postoperative radiotherapy is necessary when treating in situ ductal carcinoma with breast conserving surgery(8,10). Such surgery is considered the standard for the initial stages (I and II). A meta-analysis of clinical trials of breast cancer in the initial stages, conducted by the Early Breast Cancer Trialists' Collaborative Group, revealed the importance of radiotherapy after lumpectomy, showing that irradiation reduced the five-year local recurrence rate from 26% to 7%(13). The metaanalysis also suggested that, for every four local recurrences, one death could be avoided(14-16). Radiotherapy has evolved in the sense that it offers lower morbidity and greater efficiency in localized control. The treatment arsenal includes partial radiotherapy, intraoperative radiation therapy, the balloon technique, and intensity modulated radiation therapy. High-dose-rate (HDR) 192Ir brachytherapy with a balloon implant is frequently applied in partial radiotherapy, using the MammoSite® Radiation Therapy System. The MammoSite system consists of a catheter attached to an inflatable balloon measuring 4-6 cm in diameter, with a 192Ir source that provides, for example, 34 Gy in two daily fractions of 3.4 Gy each over five consecutive days(17,18). The balloon may be inserted in the surgical cavity during or after the breast conserving procedure. The balloons come in two sizes, 4-5 cm and 5-6 cm in diameter, and are inflated with saline solution. The balloon should be carefully adapted to the surgical cavity, considering the distance to the skin surface to ensure adequate coverage and dose homogeneity, as well as to lower the risk of complications(18). The temporary interstitial implant technique with a 99mTc-filled balloon, supplied with 99Mo/99mTc generators, was proposed by the Núcleo de Radiações Ionizantes (NRI, Center for the Study of Ionizing Radiation) at the Universidade Federal de Minas Gerais (UFMG, Federal University of Minas Gerais). This technique is justified by the availability of soluble 99mTc supplied with 2.0 Ci generators, for example. Elution can generate 2.0-3.0 mL of an aqueous solution of sodium pertechnetate [Na(TcO4)-], with sufficient activity for treatment. Furthermore, it allows for successive fractional applications until the established reference doses are achieved. This procedure may be associated with the breast conserving surgery with exposure taking place in the postoperative phase, before radiotherapy. Studies with 99mTc balloons were carried out together with the NRI brachytherapy research using 166Ho radioactive seed implants(19-24). A three-dimensional simulation of nuclear particle transportation aims to eliminate the deficiencies in two-dimensional planning by means of an analytical method in homogeneous medium, and is an important tool in improving the quality of radiotherapy procedures in oncology(25-27). Computational methods have been highly relevant for dosimetric evaluation using heterogeneous models(26,27). The Siscodes (a computational system for neutron/photon dosimetry based on stochastic methods) is a tool used for the construction of computational models and simulation in radiotherapy using stochastic codes, such as the Monte Carlo N-Particle Code (MCNP)(27-29). This system allows the conversion of computed tomography images to a voxel model. Using a database with chemical composition of tissues and nuclear data, the Siscodes associates nuclear and chemical data to the model voxels, by selecting the tissue of each voxel, as well as positioning the teletherapy and brachytherapy sources. The system uses the MCNP for simulating nuclear particle transport in the model. The resulting dosimetric data are presented in the form of spatial distribution of doses and dose-volume histograms(27). Dosimetric intercomparison is possible when similar exposure conditions are used. This objective of this article was to create such conditions, using computational methods, in order to compare and analyze two protocols-a preclinical protocol, without clinical experimentation; and a clinical practice protocol-a comparison that could not be reproduced in real-life clinical situations. This was a dosimetric inter-comparison of HDR 192Ir-filled balloons and 99mTc-filled balloons in temporary breast implants for brachytherapy, simulated under similar conditions and generating dose rate spatial distributions normalized by the source activity and accumulated dose at equivalent reference points. The hypothesis of this study was that both protocols would produce equivalent doses under pre-established conditions of activity and exposure. MATERIALS AND METHODS HDR 192Ir-filled balloon protocol A MammoSite-type applicator and centered catheter, located in the upper outer quadrant of the breast, was simulated. The study considered a 4 cm-diameter saline-filled balloon and a 5 mm-long, 1 mm-diameter metallic cylindrical linear segment, filled with 192Ir, in the (1,0,0) direction. 99mTc-filled balloon protocol A 99mTc-filled balloon implant was simulated. The source was defined as a 1.6 cm-diameter sphere, with centers coinciding with a balloon located 3.0 cm from the skin. The emissions were considered uniformly distributed throughout the volume of the balloon. Radioactive sources The characteristics of the nuclear emissions of the radionuclides 192Ir and 99mTc, in terms of activity and emission percentages, were adopted according to the Evaluated Nuclear Structure Data Files of the Medical Internal Radiation Dose Committee(30-32). Simulator Twenty-three sequential magnetic resonance imaging (MRI) scans (morphology phase) of the breast of a young patient were selected, comprising 146 slices of 4 mm each. The images were digitalized and combined to form a gray-scale voxel data model. With the Siscodes(27-29), a 2 × 2 × 2 mm3 voxel model of the breast was created. The anatomical structures were identified in each of the 23 planes, creating a three-dimensional voxel structure. From each image plane, a two-dimensional matrix was created in shades of gray, and the various matrices were combined to form the three-dimensional voxel model. One voxel, volumetrically equivalent to a tissue and identified by a specific color, was associated with each cubic element of the matrix. The chemical composition and density of the tissues were in accordance with the ICRU-44(33). Computational codes The Siscodes was used in order to generate a file containing all of the information of the computational model, in a format accepted by the MCNP5 program (version 5.2). The energy deposition in the voxels was evaluated in MeV.g-1) per transition (t). The energy deposition units (MeV.g-1.t-1) were transformed into absorbed dose rates based on the activity (cGy.h-1.mCi-1) present in the balloon (99mTc) or in the source segment (192Ir), with a conversion factor of 2133.86. After simulations, the results were incorporated into the Siscodes, generating the spatial distributions of dose. The initial seed activity was calculated in order to generate the target dose in the tumor bed. Uncertainties The computational uncertainties were analyzed for each voxel by using the MCNP5, depending on the number of executed particles. The uncertainties were less than 5% in the breast tissue and were even lower in the voxels closer to the radiation source. RESULTS The model was produced from 23 MRI slices of the mammary gland. A 21 × 60 × 23 element matrix, totaling 28,980 cubic elements or voxels, was generated. The tissue voxel model allowed a three-dimensional structure of the breast to be designed. The chemical composition of the tissues is shown in Table 1. 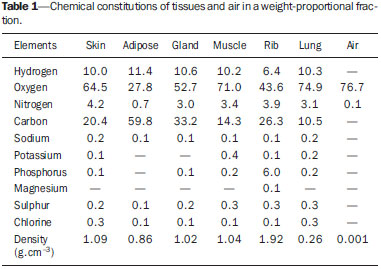 Figures 1 and 2 show the results of the temporary implant protocol simulations with a 99mTc-filled interstitial balloon and with a 192Ir-filled segment, mimicking a balloon brachytherapy booster. 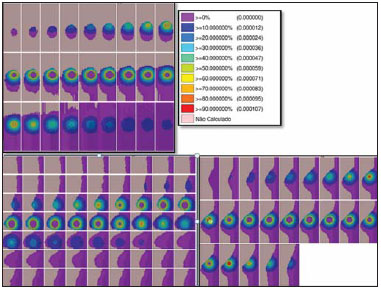 Figure 1. Spatial distribution of normalized dose rate in relation to the activity, shown in lateral, sagittal, and axial slices, induced by brachytherapy with a 192Irfilled balloon. 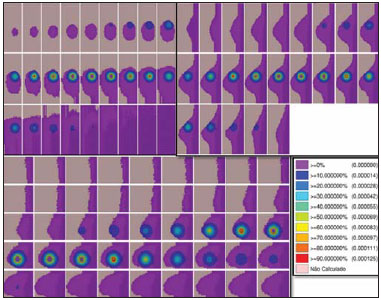 Figure 2. Spatial distribution of normalized dose rate in relation to the activity, shown in lateral, sagittal, and axial slices, induced by implantation of a balloon with a homogeneously distributed source of 99mTc. HDR 192Ir-filled temporary balloon implant Figure 1 illustrates the spatial distribution of a normalized dose in relation to the total activity of the 192Ir-filled balloon. Combined lateral, sagittal, and axial sections with dose values higher than 10% are shown. The dose was administered by the discrete 192Ir source, inserted in the intracavitary balloon. In the simulation conditions imposed, the maximum normalized dose rate reached 0.499 cGy.h-1.mCi-1. According to the distribution of doses in Figure 1, it is possible to predict the dose away from the balloon surface. 99mTc-filled temporary balloon implant Figure 2 illustrates the spatial distribution of a normalized dose in relation to the total activity of the 99mTc-filled balloon. Combined lateral, sagittal, and axial sections with dose values higher than 10% are shown. The dose was administered from an aqueous solution-distributed, homogeneous Na(TcO4)- source. The simulated balloon measured 16 mm in diameter. In this case, the maximum normalized dose rate reached 0.428 cGy.h-1.mCi-1. Table 2 shows the dosimetric values in the simulations of both protocols. The maximum dose rate (MDR) was multiplied by the dose percentage (DF) factor in relation to the reference point (RP), that is, at 8-10 mm from the balloon surface; then by the total activity at the source and by the accumulation factor (AF) of the exposure time (T), thus producing the accumulated dose at the RP. With the definition of the number of fractions (FR), the accumulated dose for the protocol at the RP was found. The 99mTc-filled balloon had 1 Ci of Na(TcO4)- and an RP at a distance of 10 mm from the balloon surface, within 25% of the MDR. For a T of 24 hours, the AF was 0.812. The HDR 192Ir-filled balloon, on the other hand, had 10 Ci of 192Ir, at the same RP, and the DF was therefore 30% of the MDR. A T of 0.4 hours produced an AF of 0.4. Under these conditions, the accumulated doses at the RP were 10.14 Gy for the 99mTc-filled balloon and 5.14 Gy for a single fraction of the HDR 192Ir-filled balloon, which will produce an equivalent dose of 10.28 Gy in two sections. DISCUSSION In this study, we have shown that a boost of 10 Gy, complementary to teletherapy, can be produced in both protocols, at the chosen reference positions. There were no statistical differences between the 99mTc-filled and 192Ir-filled balloons, in terms of the accumulated doses, considering the 5% variation given by the simulation. In this case, 99mTc-filled and 192Ir-filled balloons, in the target conditions of activity and exposure, produced equivalent absorbed doses, under the proposed conditions. The dosimetric intercomparison between the techniques was differentiated by the spatial distribution of the source and by the diameter of the balloon. This simulated condition refers to resected, in situ tumors in the initial stages. Therefore, the partial volume of the resected tumor is filled by the balloon, which may be inflated, occupying the cavity and expanding the adjacent tissue. The dose in the tumor bed, in this case, was represented by the dose in the voxels at the edges of the balloon. The 99mTc-filled balloon implant may be recommended for stage T1 and T2 tumors with a 1 cm margin, in the usual post-lumpectomy cavity(7-12). Radiation attenuation due to the radial distance is greater for the 99mTc-filled balloon than for the 192Ir-filled balloon, because 99mTc has lower energy emissions than does 192Ir(30,32). Consequently, the radial dose profile will become more restricted towards the surgical cavity, as may be observed in Figure 2 (99mTc), in comparison to Figure 1 (192Ir). Therefore, the lungs and heart are expected to receive smaller doses with the 99mTc-filled balloon than with the 192Ir-filled balloon, thus exerting fewer deleterious effects on those healthy organs. At the tumor bed, the spatial distribution of the dose from the HDR 192Ir-filled balloon showed a MDR per unit of activity equivalent to that of interstitial brachytherapy with the 99mTc-filled balloon. The MDR per unit of activity are comparable. However, because the HDR maintains the 192Ir activity at a level 3.5-5.0 times greater than that of the 99mTc, the dose rate will increase in the same proportion. Therefore, at 8 mm from the balloon, the dose rate for 1.0 Ci of 99mTc will be 1.07 Gy.h-1, reaching 0.13 Gy.h-1 in 18 hours, whereas the dose rate for 10.0 Ci of 192Ir remains practically constant for a period of 25 minutes, equal to 12.83 Gy.h-1 (12 times greater than the initial dose rate for the 99mTc-filled balloon). The lower rate, however, may reduce the deleterious effects on healthy tissues, offering better repair of the inherent sublethal damage to normal cells. Generators of 99Mo/99mTc are normally distributed with 2.0 Ci. Therefore, the 99mTc-filled balloon implant technique could be immediately incorporated without additional costs, with the support from nuclear medicine facilities. The necessary radiation safety procedures for the use of high activity (7-10 Ci) 192Ir sources, which have a half-life of 73.8 days, should be stricter than those employed for 99mTc generators. Consequently, greater management complexity is expected from the use of 192Ir, considering acquisition, transportation, and substitution of the cylindrical radiation source every three months at the centers that offer HDR brachytherapy, particularly due to the need to import the devices and employ specialized teams to replace their sources. A normal protocol for conventional radiotherapy involves the application of 25 sessions of 1.8-2.0 Gy per day, with two parallel opposed fields of 6 MV, for example, 5 days a week, for 45-60 days, with accumulated doses of 50 Gy covering the mammary gland tissue(5). For boosts in HDR 192Ir brachytherapy, maximum exposure is achieved in sessions of 20-25 minutes, compared with 24 hours for the 99mTc-filled balloon technique. Longer periods of exposure translate to greater patient discomfort. A 10-Gy boost dose at the tumor bed using the 99mTc-filled balloon technique requires 24 hours of exposure, plausibly generating such discomfort. The technique, however, may be applied postoperatively in a single fraction, the balloon being implanted immediately after local excision and exposure taking place during the recovery period. If the activity injected is doubled, the exposure time is reduced by half, or a multifraction protocol involving 4-6 hours of exposure per day, for 3 to 4 days, may be implemented, in which the radioactive liquid is replaced with water during the periods between exposures, and the radioactive solution is injected with constant activity at every application. The prescribed dose for the tumor bed should be previously defined, in order to establish the levels of activity and exposure. In well-established studies, with long-term follow-up, conducted in Europe and the United States, brachytherapy with a HDR 192Ir-filled balloon has been proven to be a safe and efficient method, with low rates of local recurrence(13-17). However, its application is limited because of the complexity of the procedure and the long (73.8 day) half-life of high-activity 192Ir sources. In addition, there are contraindications that can interfere with dosage planning(34): insufficient distance between the tumor and the skin; and an extensive cavity. Its use is not indicated when the tumor cavity is in an area of tissue that cannot be sufficiently covered(35). Undesirable anatomic surgical conditions and ineligible histological conditions can also preclude the application of the balloon technique(17). With a smaller balloon and a nuclide that provides greater dose attenuation, ineligible clinical conditions, due to inappropriate or compromised anatomy with reduced coverage tissue mass, may become less relevant. In general, the 99mTc-filled balloon technique is accessible, has a shorter learning curve, presents less complex dosimetry, and is widely available, considering the 99Mo/99mTc generators at nuclear medicine facilities in Brazil. CONCLUSION Temporary 99mTc-filled balloon implants could represent an attractive option for adjuvant radiotherapy in breast cancer. The technique supplies an adequate boost dose, with spatial dose distribution contained within the tumor bed surroundings, and its use is justified by its availability and economic viability. Acknowledgments We are grateful to the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), the Fundação de Amparo à Pesquisa do Estado de Minas Gerais (Fapemig), and the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (Capes), for providing masters and doctoral research grants, as well as research grants to the Center for the Study of Ionizing Radiation at the Universidade Federal de Minas Gerais. REFERENCES 1. IARC. Estimated cancer incidence, mortality and prevalence worldwide in 2012. International Agency for Research on Cancer, World Health Organization. [cited 2014 June 12]. Available from: globocan.iarc.fr. 2. Brasil. Ministério da Saúde. Instituto Nacional de Câncer José Alencar Gomes da Silva (INCA). Estatísticas do câncer: vigilância do câncer e fatores de risco. [cited 2014 June 12]. Available from: http://www.inca.gov.br. 3. GodinhoI ER, Koch HA. Rastreamento do câncer de mama: aspectos relacionados ao médico. Radiol Bras. 2004;37:91-9. 4. Roychoudhuri R, Evans H, Robinson D, et al. Radiation-induced malignancies following radiotherapy for breast cancer. Br J Cancer. 2004;91:868-72. 5. Purdy JA. Three dimensional physics and treatment planning. In: Perez CA, Brady LW, editors. Principles and practice of radiation oncology. 3rd ed. Philadelphia, PA: Lippincott-Raven; 1998. p. 343-70. 6. Emami B, Graham MV, Michalski JM, et al. Three dimensional conformal radiation therapy: clinical aspects. In: Perez CA, Brady LW, editors. Principles and practice of radiation oncology. 3rd ed. Philadelphia, PA: Lippincott-Raven; 1998. p. 371-86. 7. Tiezzi DG. Cirurgia conservadora no câncer de mama. Rev Bras Ginecol Obstet. 2007;29:428-34. 8. Fisher B, Costantino J, Redmond C, et al. Lumpectomy compared with lumpectomy and radiation therapy for the treatment of intraductal breast cancer. N Engl J Med. 1993;328:1581-6. 9. Glinski B, Zabek M, Mitus M. Brachytherapy boost in women with early stage breast cancer treated with breast conserving therapy. Rep Pract Oncol Radiother. 2007;12:47-51. 10. Barroso ACSD, Barbosa EM, Gebrim LH, et al. Diagnóstico e tratamento do câncer de mama. In: Projeto Diretrizes. Brasília, DF: Associação Médica Brasileira, Conselho Federal de Medicina; 2001. [cited 2014 June 12]. Available from: www.projetodiretrizes.org.br/projeto_diretrizes/024.pdf. 11. Schwartz GF, Solin LJ, Olivotto IA, et al. The consensus conference on the treatment of in situ ductal carcinoma of the breast. Breast J. 2000;6:4-13. 12. Veronesi U, Salvadori B, Luini A, et al. Breast conservation is a safe method in patients with small cancer of the breast. Long-term results three randomized trials on 1973 patients. Eur J Cancer. 1995;31A:1567-9. 13. EBCTCG (Early Breast Cancer Trialists' Collaborative Group), McGale P, Taylor C, et al. Effect of radiotherapy after mastectomy and axillary surgery on 10-year recurrence and 20-year breast cancer mortality: meta-analysis of individual patient data for 8135 women in 22 randomised trials. Lancet. 2014;383:2127-35. 14. Clarke M, Collins R, Darby S, et al. Effects of radiotherapy and of differences in the extent of surgery for early breast cancer on local recurrence and 15-year survival: an overview of the randomised trial. Lancet. 2005;366:2087-106. 15. Early Breast Cancer Trialists' Collaborative Group (EBCTCG), Darby S, McGale P, et al. Effect of radiotherapy after breast-conserving surgery on 10-year recurrence and 15-year breast cancer death: meta-analysis of individual patient data for 10,801 women in 17 randomised trials. Lancet. 2011;378:1707-16. 16. Early Breast Cancer Trialists' Collaborative Group (EBCTCG). EBCTCG Secretariat. Clinical Trial Service Unit (CTSU). Richard Doll Building. Oxford OX3 7LF. UK. 2014. 17. Dickler A, Kirk MC, Chu J, et al. The MammoSite breast brachytherapy applicator: a review of technique and outcomes. Brachytherapy. 2005;4:130-6. 18. Douglas A. Accelerated partial breast irradiation: a change in treatment paradigm for early stage breast cancer. J Surg Oncol. 2003;84:185-91. 19. Valente ES, Campos TPR. Gamma spectrometry and chemical characterization of ceramic seeds with samarium-153 and holmium-166 for brachytherapy proposal. Appl Radiat Isot. 2010;68:2157-62. 20. Valente ES, Cuperschmid EM, Campos TPR. Evaluation of HeLa cell lineage response to beta radiation from holmium-166 embedded in ceramic seeds. Braz Arch Biol Technol. 2011;54:957-64. 21. Nogueira LB, Campos TPR. Nuclear characterization and investigation of radioactive bioglass seed surfaces for brachytherapy via scanning electron microscopy. J Sol-Gel Sci Technol. 2011;58:251-8. 22. Nogueira LB, Campos TPR. Radiological response of ceramics and polymeric devices for breast brachytherapy. Int J Appl Radiat Isot. 2012;70:663-9. 23. Nogueira LB, Silva HLL, Campos TPR. Experimental dosimetry in conformal breast teletherapy compared with the planning system. Appl Radiat Isot. 2015;97:93-100. 24. Campos TPR, Andrade JPL, Costa IT, et al. A radioactive seed implant on a rabbit's liver following a voxel model representation for dosimetric proposals. In: 2005 International Atlantic Conference - INAC 2005. Santos, SP; 2005. 25. MCNP5. Monte Carlo Team. MCNP - A General Monte Carlo N-Particle Transport Code, Version 5. Volume I: Overview and theory. Los Alamos: Los Alamos National Laboratory; 2003. 26. Kalos MH, Whitlock PA. Monte Carlo methods. Vol. I: basics. New York: Wiley; 1986. 27. Campos TPR. Computational simulations in medical radiation: a new approach to improve therapy. [cited 2016 Jan 15]. Available from: http://www.sbmac.org.br/bol/boletim_2002/campos-4emc.pdf. 28. Trindade BM, Campos TPR. Stochastic method-based computational system for neutron/photon dosimetry applied to radiotherapy and radiology. Radiol Bras. 2011;44:109-16. 29. Trindade BM, Christóvão MT, Trindade DFM, et al. Comparative dosimetry of prostate brachytherapy with I-125 and Pd-103 seeds via SISCODES/MCNP. Radiol Bras. 2012;45:267-72. 30. International Atomic Energy Agency. Nuclear Data Services. Medical Application and Nuclear Data Section - MIRD. [cited 2015 Jan 15]. Available from: http://www.nndc.bnl.gov/mird/. 31. ENDF/BVI Decay Data. Evaluated Nuclear Data File (ENDF) ENDF/B-VII.1 Nuclear Data for Science and Technology: Cross Sections, Covariances, Fission Product Yields and Decay Data, released December 22, 2011. [cited 2013 Nov 15]. Available from: http://t2.lanl.gov/data/decayd.html. 32. Korea Atomic Energy Research Institute. Electron and photon attenuation. [cited 2012 Nov 19]. Available from: http://atom.kaeri.re.kr:8080/ex.html. 33. International Commission on Radiation Units and Measurements. ICRU Report 44. Tissue substitutes in radiation dosimetry and measurement. Bethesda, MD: ICRU; 1989. 34. Tuschy B, Berlit S, Romero S, et al. Clinical aspects of intraoperative radiotherapy in early breast cancer: short-term complications after IORT in women treated with low energy x-rays. Radiat Oncol. 2013;8:95. 35. Ravi A, Lee S, Karsif K, et al. MammoSite multilumen catheter: dosimetry considerations. J Cancer Res Ther. 2011;7:64-8. 1. Postdoctoral Fellow, Professor in the Department of Nuclear Engineering, Universidade Federal de Minas Gerais (UFMG), Belo Horizonte, MG, Brazil 2. MD, Nuclear Medicine Physician, Doctoral Student in the Graduate Program in Nuclear Sciences and Techniques, Núcleo de Radiações Ionizantes (NRI) at the Universidade Federal de Minas Gerais (UFMG), Belo Horizonte, MG, Brazil 3. PhD, Docent at the Center for the History of Medicine, Universidade Federal de Minas Gerais (UFMG), Belo Horizonte, MG, Brazil Mailing address: Dr. Tarcísio Passos Ribeiro de Campos Departamento de Engenharia Nuclear, Universidade Federal de Minas Gerais Avenida Antônio Carlos, 6627, Campus UFMG, PCA 1 – Anexo Engenharia Belo Horizonte, MG, Brazil, 31270-010 E-mail: tprcampos@pq.cnpq.br Received January 27, 2015. Accepted after revision May 26, 2015. Study conducted in the Department of Nuclear Engineering, Universidade Federal de Minas Gerais (UFMG), Belo Horizonte, MG, Brazil. |
|
Av. Paulista, 37 - 7° andar - Conj. 71 - CEP 01311-902 - São Paulo - SP - Brazil - Phone: (11) 3372-4544 - Fax: (11) 3372-4554