Radiologia Brasileira - Publicação Científica Oficial do Colégio Brasileiro de Radiologia
AMB - Associação Médica Brasileira CNA - Comissão Nacional de Acreditação
 Vol. 48 nº 3 - May / June of 2015
Vol. 48 nº 3 - May / June of 2015
|
CASE REPORT
|
|
Hybrid treatment of penetrating aortic ulcer |
|
|
Autho(rs): Juan Antonio Herrero Lara1; Daniela de Araújo Martins-Romêo1; Carlos Caparrós Escudero1; Rosa María Lepe Vázquez2; María del Carmen Prieto Falcón3; Vinicius Bianchi Batista3 |
|
|
Keywords: Computed tomography; Thoracic aorta; Aortic diseases; Atherosclerosis; Cardiovascular surgery. |
|
|
Abstract: INTRODUCTION
Penetrating aortic ulcer (PAU) is an atherosclerotic plaque complication involving injury to the aortic wall with clinical manifestation of chest pain. It is one of the conditions included in the acute aortic syndrome, representing a medical emergency with potential complications that may lead to aortic rupture(1,2). In the setting of penetrating aortic ulcer, the radiologist's role is critical since this disease is not frequently observed and can hardly be suspected clinically, so an erroneous or late diagnosis may lead to high rates of morbidity and mortality. In such a context, computed tomography (CT) is the diagnostic imaging method of choice. The authors report a case of PAU in an uncommon location (aortic arch), starting with chest pain and evolving with dysphonia. The patient was submitted to a recently implemented endovascular surgical treatment. CASE REPORT A 65-year old, male, obese patient who was formerly smoker and presented with chronic obstructive pulmonary disease at GOLD IV stage (severely decreased air flow with risk to the patient's life in case of temporary clinical worsening), with history of hypertensive and ischemic heart disease. The initial clinical manifestation was sharp precordial pain radiating to the left brain and respiratory difficulty. Cardiac enzymes test results were normal and electrocardiography did not present any ventricular repolarization alteration. Cardiac catheterization did not demonstrate any relevant lesion. The patient evolved with sudden dysphonia onset and bronchospasm. Chest CT established the diagnosis of PAU in the aortic arch. The patient was referred for emergency endovascular surgical treatment with successful outcome. DISCUSSION Male sex, advanced age and hypertension constitute the main acute aortic syndrome related risk factors(2,3), which in only 5% of cases is justified by PAU, an uncommon, underdiagnosed condition, making radiologists' work more difficult. PAU refers to ulceration of an atheromatous plaque, deeply penetrating the intima into the aortic media, causing hemorrhage(4). The evolution of this condition is variable: in some cases, it is limited to the aortic media due to the fibrotic component of the atheromatous plaque, with development of a small, circumscribed area of dissection or ulceration; in other cases, the lesion extends up to a distal point of the aortic lumen re-entry causing dissection. Another possibility is that the lesion reaches the adventitia causing a pseudoaneurysm. In more extreme cases, the lesion may cross the wall with aortic rupture(1,4). Location of PAU in the aortic arch is less frequently found than in the descending aorta, because of the close relationship between PAU and atherosclerosis that is most prevalent in the descending aorta, and because of the high-velocity blood flow that becomes a protective factor in the ascending aorta and aortic arch(4). CT is the technique of choice in the evaluation of acute aortic syndromes because of its wide availability, rapid images acquisition, reproducibility, high sensitivity and specificity. It is recommended that a non-contrast-enhanced image acquisition be initially performed to identify possible associated hematomas and distribution of parietal calcifications, followed by an acquisition in the arterial phase after iodinated contrast injection. At contrast-enhanced images a focal alteration is identified in the aortic contour, corresponding to the ulcer that may be associated with mural hematoma and parietal enhancement due inflammation and hyperemia (Figures 1 and 2). The presence of the hematoma is useful to differentiate a PAU from a common or uncomplicated ulcer. However, one must consider the presence of clinical manifestation of pain due to the high incidence of parietal irregularities in patients at advanced ages that may show a similar radiological appearance (Figure 3). In some cases like the present one, symptoms resulting from the recurrent compression of the laryngeal nerve by the hematoma may be observed. Such symptoms include dysphonia, dysphagia or superior vena cava syndrome. 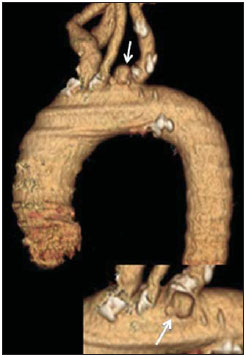 Figure 1. 3D reconstruction, volume-rendered image. Digitform or collar button image deforming the expected contour of the aortic arc wall between the common carotid and left subclavian arteries, compatible with PAU. 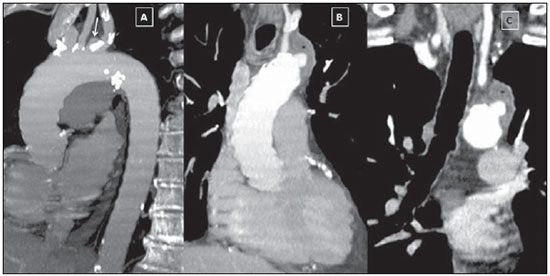 Figure 2. Sagittal (A) and coronal obliques (B,C) maximum intensity projection reconstruction images. Surrounding hyperdensity with peripheral enhancement compatible with parietal hematoma (asterisks); sign of instability at the theoretical location of the left recurrent laryngeal nerve which explains the clinical signs of dysphonia. 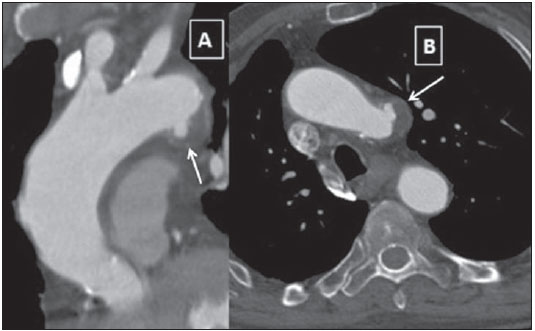 Figure 3. Sagittal oblique (A) and axial (B) maximum intensity projection reconstruction images of other patient in whom a common and uncomplicated ulcer was incidentally diagnosed during the investigation of other non-related disease. A digitform image is identified deforming the proximal descending aorta contour. The surrounding hypodense image is compatible with thrombotic material and not a mural hematoma. PAU presents radiological similarity with pseudoaneurysm, a lesion of traumatic origin that causes intima-media rupture, with aortic rupture contained by the adventitia. However, in the present case, there was no previous history of trauma, intimal flap image, nor the lesion was located in the aortic isthmus (that is the usual site of pseudoaneurysms, due to the fixation effect secondary to the ligamentum arteriosum insertion). In addition to common ulcer and pseudoaneurysm, mycotic or infectious aneurysms should also be included in the differential diagnosis(3). In the present case, there is no clinical data supporting a possible infectious cause. The therapeutic approach to PAU is similar to the approach to type B aortic dissection that is usually conservative. However, in the present case, option was made to submit the patient to the hybrid intervention due to the location of the PAU which could lead to severe complications and due to the persistent clinical condition of the patient. The patient presented with advanced chronic obstructive pulmonary disease and other comorbidities which raised the surgical risk, and for this reason the hybrid endovascular surgical technique was adopted(5) instead of complete aortic arch replacement (Figure 4). 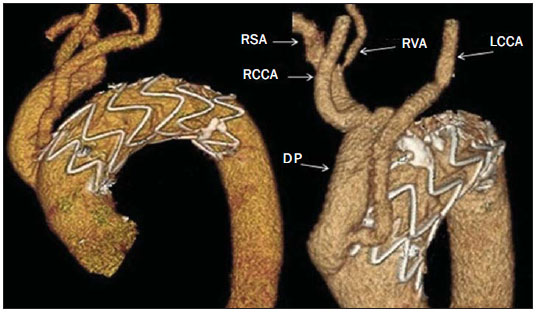 Figure 4. Post-treatment CT. 3D reconstruction, volume-rendered image. The supra-aortic trunks are preserved by means of debranching with a bifurcated Dacron tube graft (Dacron prosthesis – DP) from the ascending aorta to the brachiocephalic arterial trunk and left common carotid artery (LCCA), followed by endovascular (femoral approach) placement of a prosthesis in the aortic arch, with satisfactory evolution observed at threemonth follow-up. RSA, right subclavian artery; RCCA, right common carotid artery; RVA, right vertebral artery. Recently, several studies demonstrated the endovascular treatment safety and effectiveness in cases of thoracic aorta conditions, which makes this technique an interesting therapeutic alternative to reduce morbidity and mortality(6,7). The possibility of combining this technique and surgery in a single procedure allows for the treatment of a greater number of patients. After the intervention, six-month and twelve-month CT follow-up followed by annual follow-up is recommended to confirm the correct ulcer exclusion and to rule out possible stent complications such as migration, infraexpansion or endoleakage(8). REFERENCES 1. Chin AS, Fleischmann D. State-of-the-art computed tomography angiography of acute aortic syndrome. Semin Ultrasound CT MR. 2012;33:222-34. 2. Vilacosta I, Román JA. Acute aortic syndrome. Heart. 2001;85:365-8. 3. Birchard KR. Acute aortic syndrome and acute traumatic aortic injuries. Semin Roentgenol. 2009;44:16-28. 4. Macura KJ, Corl FM, Fishman EK, et al. Pathogenesis in acute aortic syndromes: aortic dissection, intramural hematoma, and penetrating atherosclerotic aortic ulcer. AJR Am J Roentgenol. 2003;181:309-16. 5. Iida Y, Kawaguchi S, Koizumi N, et al. Thoracic endovascular aortic repair with aortic arch vessel revascularization. Ann Vasc Surg. 2011;25:748-51. 6. Scali ST, Goodney PP, Walsh DB, et al. National trends and regional variation of open and endovascular repair of thoracic and thoracoabdominal aneurysms in contemporary practice. J Vasc Surg. 2011;53:1499-505. 7. Lioupis C, Abraham CZ. Results and challenges for the endovascular repair of aortic arch aneurysms. Perspect Vasc Surg Endovasc Ther. 2011;23:202-13. 8. Johnson PT, Black JH, Zimmerman SL, et al. Thoracic endovascular aortic repair: literature review with emphasis on the role of multidetector computed tomography. Semin Ultrasound CT MR. 2012;33:247-64. 1. MDs, Radiologists at Unidade de Gestão Clínica (UGC) de Diagnóstico por Imagem - Hospital Universitario Virgen Macarena, Sevilha, Spain 2. MD, Radiologist at Unit of Radiodiagnosis - Hospital Nuestra Señora de la Merced, Osuna, Sevilha, Spain 3. MDs, Residents, Unidade de Gestão Clínica (UGC) de Diagnóstico por Imagem - Hospital Universitario Virgen Macarena, Sevilha, Spain Mailing Address: Dr. Juan Antonio Herrero Lara U.G.C. de Diagnóstico por Imagem - Hospital Universitário Virgen Macarena Avd. Dr. Fedriani, 3 41007 Sevilha, Spain E-mail: jaherrero5@hotmail.com Received August 28, 2014. Accepted after revision January 9, 2015. Study developed at Unidade de Gestão Clínica (UGC) de Diagnóstico por Imagem - Hospital Universitario Virgen Macarena, Sevilha, Spain. |
|
Av. Paulista, 37 - 7° andar - Conj. 71 - CEP 01311-902 - São Paulo - SP - Brazil - Phone: (11) 3372-4544 - Fax: (11) 3372-4554