Radiologia Brasileira - Publicação Científica Oficial do Colégio Brasileiro de Radiologia
AMB - Associação Médica Brasileira CNA - Comissão Nacional de Acreditação
 Vol. 47 nº 6 - Nov. / Dec. of 2014
Vol. 47 nº 6 - Nov. / Dec. of 2014
|
ORIGINAL ARTICLE
|
|
Dosimetry of patients submitted to cerebral PET/CT for the diagnosis of mild cognitive impairment |
|
|
Autho(rs): Priscila do Carmo Santana1; Arnaldo Prata Mourão2; Paulo Márcio Campos de Oliveira1; Felipe Dias Bernardes3; Marcelo Mamede4; Teógenes Augusto da Silva5 |
|
|
Keywords: Dosimetry; PET/CT; Mild cognitive impairment. |
|
|
Abstract: INTRODUCTION
Patients with mild cognitive impairment (MCI) may have progression of their clinical condition Alzheimer's disease. Such patients present with a memory deficit similar to that in Alzheimer's disease, without other features of that disease, but the rate of conversion to Alzheimer's disease is approximately 10% to 15% per year. Alzheimer's disease is a neurodegenerative disease most frequently associated with the aging process, whose cognitive and neuropsychiatric manifestations result in progressive deficiency and eventual and eventual incapacitation(1,2). Between 60% and 70% of the patients presenting with MCI also present with moderate to severe hypometabolism in the association cortex. Alzheimer's patients tend to present with hypometabolism in the parietal and temporal lobes and in the posterior cingulum. For that reason, in addition to clinical investigation, positron emission tomography/computed tomography (PET/CT) with theutilization of the radiopharmaceutical 18F-fluorodeoxyglucose (18F-FDG) is one of the techniques for early diagnosis of MCI and Alzheimer's disease. PET is the most sensitive and specific imaging method in the detection of metabolic changes, but its spatial resolution is limited. Computed tomography (CT), therefore, is helpful in the anatomical identification of possible changes which may be found at PET/CT images. As a PET/CT scan is performed, the patient is submitted to radiation coming from the radiopharmaceutical injection as well as to the x-ray dose originated from the CT apparatus. For that reason, when compared with other diagnostic imaging modalities, such a technique submits the patients to higher effective doses than other imaging techniques do(3-5). Dosimetry is the quantitative study of the effects caused by ionizing radiation on a medium. The way by which ionizing radiations interact with an absorbing medium depends upon its composition and the radiation energy, therefore it is very important to know the characteristics of the incident radiation beam as well as the quantification of the energy deposited by such a beam on a given material(6). Dosimetry may be performed on patients either directly or indirectly. When directly performed, thermoluminescent dosimeters are utilized. Such dosimeters are small crystals that have the capability of storing energy from radiation and release it under the form of light as they are heated to certain temperatures. The effective atomic number of lithium fluoride thermoluminescent dosimeters is very close to that of human tissue, so their positioning on the patient or on phantoms within the direct radiation beam does not impair the image acquisition for the energy range utilized in conventional radiodiagnosis(6). Dosimetry studies can be indirectly performed by means of biokinetic models of radionuclide incorporation and the knowledge on the interaction of radiation with matter. The study of absorbed and effective radiation doses on patients submitted to PET/CT for cancer diagnosis and staging is important to allow for the optimization of radiodiagnostic procedures and to assure the application of radiological protection principles. Thus, it is possible to obtain diagnostic quality images with the lowest possible patient exposure to radiation. With the purpose of knowing the effective dose to which such patients are submitted, the present study is aimed at determining the radiation levels with each one of the modalities in the most radiosensitive organs, such as the crystalline and the thyroid gland, which may be directly exposed to radiation emitted by the CT apparatus and from the radiopharmaceutical injection. MATERIALS AND METHODS A Discovery 690 64-channeI PET/CT apparatus was utilized in order to determine the effective and absorbed doses resulting from CT. The following technical parameters were utilized for cerebral images acquisition: voltage = 120 kV; current = 150 mA (without modulation); slice thickness = 3.75 mm; beam thickness = 20 mm; pitch = 0.531; table velocity = 10.62 mm/rotation; exposure time per rotation = 0.5 s. For the measurement of absorbed and effective dose from PET/CT it was necessary to ensure appropriate working conditions of the equipment, by means of application of quality control tests recommended by the manufacturer, such as x-ray tube heating and calibration and control of detectors' response homogeneity and reproducibility. Two steps were necessary to determine the effective dose from the PET/CT scans, as follows: one to determine contribution to effective dose caused by CT and another to evaluate the contribution coming from the radiopharmaceutical activity. The absorbed and effective dose received by the patients during CT scans were measured by means of thermoluminescent detectors (TLDs) inserted into Alderson Randon® anthropomorphic phantoms (male and female versions). The previously selected and calibrated rod-shaped (cylindrical) dosimeters utilized in this experiment were based on lithium fluoride activated with magnesium and titanium (LiF: Mg,Ti – TLD-100), and made by Harshaw Chemical Company. The detectors calibration was performed in the energy range of interest given by the reference radiation for CT at the calibration laboratory of Centro de Desenvolvimento da Tecnologia Nuclear (CDTN) (Center for Development of Nuclear Technology), identified as RQT9, produced with a voltage of 120 kV and 8.4 mm aluminum half-value layer. Figure 1 shows the anthropomorphic phantom positioned in the gantry for the performance of the tomography procedure, where one can observe five capsules positioned in the region of the breasts, thyroid and crystalline. 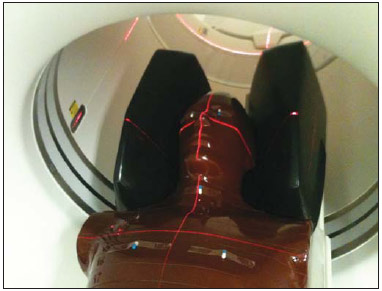 Figure 1. Positioning of the male anthropomorphic phantom for the performance of the PET/CT scan. The TLD-100 detectors were inserted into the Alderson Randon® anthropomorphic phantoms at each point corresponding to the most radiosensitive organs and with greater probability of exposure due to their insertion in the irradiation field or proximity to it (brain, breast, thyroid, crystalline). Several points were selected for each organ based on its volume, and for each selected point, three encapsulated detectors were inserted in such a way to increase the metrological reliability of the measurements. After preparation of the anthropomorphic phantoms, they were submitted to the same image acquisition protocol utilized with patients, and an irradiation was performed. In order to evaluate cerebral metabolic changes, the CT scan was restricted to the protocol for brain images acquisition, therefore only that region directly received the primary x-ray beam. Once the absorbed dose results were obtained for each organ, the effective doses in the CT scans were calculated by means of equation 1: 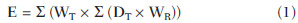 where: DT represents the mean value of the results of the absorbed dose readings by the dosimeters positioned in the tissue or organ "T"; WR represents the weighting factor for the type of radiation "R" given by the ICRP 103 publication, which, for x-radiation is the unit(7); WT represents the weighting factor for the tissue or organ "T" given by the ICRP 103 publication(7). In the PET modality, the injected radiopharmaceutical (18F-FDG) is distributed throughout the entire body, but it has greater affinity with organs with a high glucose uptake, such as the brain and the heart, besides excretory organs such as the bladder. Therefore, the radiopharmaceutical will contribute with higher radiation levels in several organs outside of the evaluated region. In order to determine such a contribution, the model proposed by IRCP 106(8-10) was utilized. The model proposed by the IRCP 106 publication for the radiopharmaceutical 18F-FDG is based on scientific studies presenting biokinetic models after its intravenous radiopharmaceutical administration(11). According to that publication, there is an initial 18F-FDG absorption in the heart (4%), brain (8%), liver (5%), lungs (3%) and all the other tissues (80%). The retention in the mentioned organs of origin is considered as being infinite (without taking the delayed absorption into consideration). A 30% fraction of the activity in other organs or tissues is considered as being excreted by urine, with biological half-life of 12 minutes (elimination of 25%) and 1.5 hour (elimination of 75%), according to the biokinetic model for kidney-bladder. Also, according to the model proposed by IRCP 106, organs such as the crystalline and thyroid present insignificant uptake as compared with others, as such organs present low glucose metabolism. In the PET scans for identification of MCIs, the calculation of the radioactive activity to be incorporated by the patient is based on the patient´s body mass index. A factor of 3.7 MBq/kg was utilized for each patient. The data from 59 female patients and 38 male patients submitted to this protocol were utilized. According to the model proposed by the ICRP 106, coefficients (Γ) are utilized, allowing for the calculation of the absorbed dose value (D) in the organs and the effective dose (E) as a function of radioactive activity (A) injected into the patients, injected radionuclide (in this case fluorine-18), patient's age and organ, as per equations 2 and 3:  RESULTS The calibration coefficient for the reference radiation (RQT9) was (82.9 ± 8.3) mGy/nC. The mean effective dose originated by the PET/CT scan in the protocol for MCI diseases studies was (5.34 ± 1.99) mSv. The mean age of the patients submitted to PET/CT scans was 76 years, with a mean height of 1.57 m, weight 62.86 kg, injected activity of (295.99 ± 106.21) MBq and effective dose exclusively due to incorporation of the radiopharmaceutical of (5.22 ± 1.84) mSv, as shown on Table 1. All uncertainties were calculated for the confidence interval of 95.45% of the data, with a coverage factor k = 2. 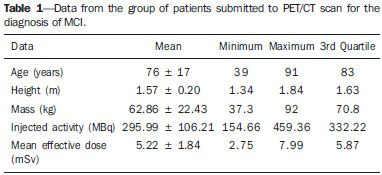 The values for mean absorbed dose in patients submitted to PET/CT resulting only from irradiation by the x-rays are shown on Table 2. 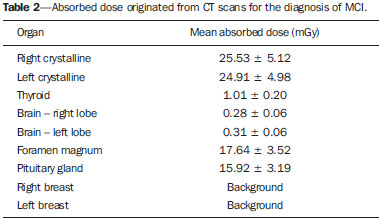 The region that received the highest CT radiation level was the crystalline, which received (25.53 ± 5.12) mGy and (24.91 ± 4.98) mGy for the right and left sides, respectively, being therefore, the most irradiated tissue or organ in this type of image acquisition protocol. The values for mean absorbed dose in patients submitted to PET/CT resulting from the incorporation of 18F-FDG are presented on Table 3. For an organ such as the brain, the absorbed dose was (11.25 ± 4.04) mGy and for the thyroid it was (2.96 ± 1.06) mGy. DISCUSSION It was possible to observe the low level of radiation in the thyroid, as the dose to this organ comes mostly from the incorporation of 18F-FDG and it is outside the irradiation field during CT image acquisition. The crystalline is a radiosensitive organ, receiving irradiation only from CT, as such organ does not significantly uptake 18F-FDG . On the other hand, the brain and the heart are organs with high blood irrigation, with increased 18F-FDG uptake and, consequently with a significant increase in the absorbed dose in such organs. In the breasts, the absorbed dose originated from cranial CT could not be statistically distinguished, as it is at the background radiation level, demonstrating that the positioning of detectors in organs even more distant from the primary radiation beam is not necessary for the presently utilized image acquisition protocol. The bladder is the organ that undergoes the greatest energy deposition for the PET/CT scans, as the urinary tract is the 18F-FDG excretion pathway and the radiopharmaceutical remains in that organ for an extended period of time. Such a fact explains the high radiation dose in such organ, representing, approximately, in relative terms, averagely 25% of the whole-body absorbed dose. The remaining organs received very similar radiation levels, representing, on average, 3% of the whole-body absorbed dose per evaluated organ. The lack of dosimetry studies approaching brain PET/CT scans does not allow for good results comparison, but, for some specific scans it is possible to obtain a relation between results(12-18). Khamwan et al. have demonstrated that for 35 Thai patients submitted to whole-body PET/CT scans for cancer, the mean effective dose was 18.85 mSv calculated by computational methods(17). Huang et al., evaluating the risk for cancer induction in the US and Hong Kong populations, have estimated the whole-body radiation dose originated from 18F-FDG and from CT scans. They determined that the doses, upon utilization of three different protocols (A, B, and C) were, respectively 13.45, 24.79 and 31.91 mSv for female patients and 13.65, 24.80 and 32.18 mSv for male patients(12). Such results demonstrate that the radiation levels, exclusively for brain scans, submit the patients to much lower radiation levels than effective doses delivered by whole-body scans, as expected. Pina et al. have evaluated the distribution of absorbed doses in organs, in three CT apparatuses, with cranial protocols, utilizing TLD-100 dosimeters inserted into an Alderson Rando®anthropomorphic phantom. Mean absorbed dose was 16 mGy for the crystalline, and 0.5 mGy for the thyroid, representing differences of respectively 52% and 50% as compared with data obtained in the present study(19). The radiation level in patients submitted to PET/CT scan in the diagnosis of MCIs is approximately 98% originated from the radiopharmaceutical incorporation, which demonstrates the importance of the injection of the radioactive element in the quantity that allows for an accurate diagnosis. Therefore, a study aimed at optimizing the activity injected into patients might be extremely efficient in the reduction of doses delivered to patients. CONCLUSION Optimized protocols for the calculation of the radioactive activity that will be injected into each patient may contribute to the reduction of the effective dose during diagnosis by PET/CT. The utilization of individualized calculation of the radiopharmaceutical is also a primary factor for the reduction of the effective dose. The knowledge about PET/CT equipment resources, awareness and training of professionals with respect to radiological protection of patients may directly affect the choice of protocols, and consequently, the dose to be delivered to such patients. REFERÊNCIAS 1. Zhao Q, Tang XC. Effects of huperzine A on acetylcholinesterase isoforms in vitro: comparison with tacrine, donepezil, rivastigmine and physostigmine. Eur J Pharmacol. 2002;455:101-7. 2. Janus C, Westaway D. Transgenic mouse models of Alzheimer's disease. Physiol Behav. 2001;73:873-86. 3. Deloar HM, Fujiwara T, Shidahara M, et al. Estimation of absorbed dose for 2-[F-18]fluoro-2-deoxy-D-glucose using whole-body positron emission tomography and magnetic resonance imaging. Eur J Nucl Med. 1998;25:565-74. 4. Hays MT, Segall GM. A mathematical model for the distribution of fluorodeoxyglucose in humans. J Nucl Med. 1999;40:1358-66. 5. Hays MT, Watson EE, Thomas SR, et al. MIRD dose estimate report no. 19: radiation absorbed dose estimates from (18)F-FDG. J Nucl Med. 2002;43:210-4. 6. Oliveira PMC. Avaliação de parâmetros da qualidade de imagem e dosimetria de pacientes submetidos a exames radiológicos de tórax. [Tese de doutorado]. Belo Horizonte, MG: Universidade Federal de Minas Gerais; 2012. 7. [No authors listed]. The 2007 Recommendations of the International Commission on Radiological Protection. ICRP Publication 103. Annals ICRP. 2007;37(2-4):1-332. 8. [No authors listed]. Radiation dose to patients from radiopharmaceuticals. A report of a Task Group of Committee 2 of the International Commission on Radiological Protection. Ann ICRP. 1987;18(1-4):1-377. 9. [No authors listed]. Radiation dose to patients from radiopharmaceuticals (addendum 2 to ICRP Publication 53). Ann ICRP. 1998;28(3):1-126. 10. ICRP. Radiation dose to patients from radiopharmaceuticals - Addendum 3 to ICRP Publication 53. ICRP Publication 106. Ann ICRP. 2008;38(1-2):1-197. 11. Mejia AA, Nakamura T, Masatoshi I, et al. Estimation of absorbed doses in humans due to intravenous administration of fluorine-18fluorodeoxyglucose in PET studies. J Nucl Med. 1991;32:699-706. 12. Huang B, Law MW, Khong PL. Whole-body PET/CT scanning: estimation of radiation dose and cancer risk. Radiology. 2009;251:166-74. 13. Al-Haj AN, Lobriguito AM, Arafah A, et al. Deriving staff and public doses in a PET/CT facility from measured radiation levels using thermoluminescent dosimetry. Radiat Prot Dosimetry. 2011;144:487-91. 14. Zenóbio MAF, da Silva TA. Absorbed doses on patients undergoing tomographic exams for pre-surgery planning of dental implants. Appl Radiat Isot. 2007;65:708-11. 15. Bedetti G, Pizzi C, Gavaruzzi G, et al. Suboptimal awareness of radiologic dose among patients undergoing cardiac stress scintigraphy. J Am Coll Radiol. 2008;5:126-31. 16. Committee to Assess Health Risks from Exposure to Low Levels of Ionizing Radiation, National Research Council. Health risks from exposure to low levels of ionizing radiation: BEIR VII Phase 2 (2006). Washington, DC: National Academy Press; 2006. 17. Khamwan K, Krisanachinda A, Pasawang P. The determination of patient dose from (18)F-FDG PET/CT examination. Radiat Prot Dosimetry. 2010;141:50-5. 18. Hillner BE, Siegel BA, Liu D, et al. Impact of positron emission tomography/computed tomography and positron emission tomography (PET) alone on expected management of patients with cancer: initial results from the National Oncologic PET Registry. J Clin Oncol. 2008;26:2155-61. 19. Pina DR, Duarte SB, Ghilardi Netto T, et al. Controle de qualidade e dosimetria em equipamentos de tomografia computadorizada. Radiol Bras. 2009;42:171-7. 1. PhD, Associate Professor, Universidade Federal de Minas Gerais (UFMG), Belo Horizonte, MG, Brazil 2. PhD, Associate Professor, Centro Federal de Educação Tecnológica de Minas Gerais (Cefet-MG), Belo Horizonte, MG, Brazil 3. Graduate Student of Technology in Radiology, Universidade Federal de Minas Gerais (UFMG), Belo Horizonte, MG, Brazil 4. Research Fellow, Full Professor, Universidade Federal de Minas Gerais (UFMG), Belo Horizonte, MG, Brazil 5. Research Fellow, Titular Researcher, Centro de Desenvolvimento da Tecnologia Nuclear – Comissão Nacional de Energia Nuclear (CDTN-CNEN), Belo Horizonte, MG, Brazil Mailing Address: Dra. Priscila do Carmo Santana Departamento de Anatomia e Imagem Avenida Professor Alfredo Balena, 190 Belo Horizonte, MG, Brazil, 30130-100 E-mail: pridili@gmail.com Received May 16, 2013. Accepted after revision April 17, 2014. Study developed at Universidade Federal de Minas Gerais (UFMG), Belo Horizonte, MG, Brazil. Financial support: Dean's Office for Research – Universidade Federal de Minas Gerais, Fundação de Amparo à Pesquisa do Estado de Minas Gerais (Fapemig), and INCT de Metrologia das Radiações. |
|
Av. Paulista, 37 - 7° andar - Conj. 71 - CEP 01311-902 - São Paulo - SP - Brazil - Phone: (11) 3372-4544 - Fax: (11) 3372-4554