Radiologia Brasileira - Publicação Científica Oficial do Colégio Brasileiro de Radiologia
AMB - Associação Médica Brasileira CNA - Comissão Nacional de Acreditação
 Vol. 47 nº 5 - Sep. / Oct. of 2014
Vol. 47 nº 5 - Sep. / Oct. of 2014
|
ORIGINAL ARTICLE
|
|
Computed tomography-guided percutaneous biopsy of bone lesions: rate of diagnostic success and complications |
|
|
Autho(rs): Macello Jose Sampaio Maciel1; Chiang Jeng Tyng2; Paula Nicole Vieira Pinto Barbosa2; Almir Galvão Vieira Bitencourt3; João Paulo Kawaoka Matushita Junior4; Charles Edouard Zurstrassen5; Wu Tu Chung6; Rubens Chojniak7 |
|
|
Keywords: Bone neoplasms; Needle biopsy; Computed tomography; Interventional radiology; Complications. |
|
|
Abstract: INTRODUCTION
Recent studies published in Brazil have highlighted the relevance of interventional radiology in the appropriate collection of specimens for the diagnosis and treatment of diseases affecting different parts of the body(1-6). Percutaneous biopsy is an important tool in the evaluation of bone lesions suspicious for malignancy. Suspicious primary bone tumors, or systemic cancer recurrence such as bone metastases constitute frequent indications for computed tomography (CT)-guided percutaneous biopsy. The presumptive decision to approach a lesion as recurrence of a known primary malignant tumor without pathological confirmation may erroneously lead to inappropriate treatment of benign diseases or even incorrect management of a second primary tumor different from the first one. The CT-guided procedure is a safe and accurate method to define the diagnosis(7-11). The diagnostic results are variable according to the location of the lesion; those observed in lesions of extremities and pelvic bones are more accurate as compared with those observed in lesions located in the vertebral column(12). The rate of complications in CT-guided bone biopsies is very low (1.1%), while in open biopsy it may be as high as 16%(13). Even considering that it is generally a low risk procedure, minor side effects and complications such as infections, fractures and bleeding may occur(14-16). A successful bone biopsy is the procedure that gets enough material for an appropriate histopathological analysis and definition of the diagnosis, i.e., a specific diagnostic result capable of guiding the requesting physician in the decision making regarding to the approach to be adopted either in relation to treatment, follow-up or discharge of the patient(17). The ideal result should be definite, with no possibility of differential diagnosis, and reliable enough to allow the physician to make a decision on the approach to be adopted. In the literature, the rate of diagnostic definition of percutaneous biopsies of musculoskeletal lesions is 69-88%(13,18-22). The present study was aimed at determining the rates of diagnostic definition and complications of CT-guided percutaneous biopsy of bone lesions suspicious for malignancy. MATERIALS AND METHODS Retrospective study developed at the Imaging Department of A.C.Camargo Cancer Center, including patients submitted to CT-guided percutaneous biopsy of bone lesions, in the period from January 2010 to December 2012. The present study followed the principles of the Helsinki Statement on Health in all Policies and was approved by the Committee for Ethics in Research of the institution. A written term of free and informed consent for the biopsy and verbal consent for inclusion of biopsy data in the study were obtained from the patients. Previously to the procedures, in all the cases, all the available CT images were reviewed, as well as bone scintigraphy, magnetic resonance imaging (MRI) and positron emission computed tomography (PET/CT) images, as available. Such images were analyzed by a radiologist who determined the most appropriate site for specimen collection with a safe approach, considering the lesion characteristics, possible complications and chances for collection of sufficient material. Before all the procedures, coagulation tests, including platelet count, prothrombin time, international normalized ratio, and partial thromboplastin time were performed. Coagulopathy cases were corrected before the procedure. The patient positioning for biopsy was based on the location of the target lesion. The selected site was confirmed by 5.0 mm-thick CT sections and a single section of the lesion was selected. The best access to the lesion was planned and drawn from the target lesion to the skin surface. The selection of depth of the lesion and the skin entrance site was based on the metal marker previously placed on the patient's skin (Figure 1). 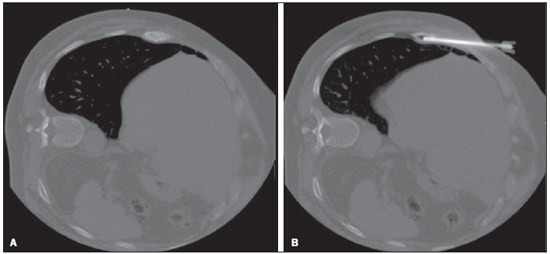 Figure 1. CT-guided percutaneous bone biopsy of a sclerotic rib lesion. Suspicion of metastasis from prostate adenocarcinoma confirmed after histopathological analysis. A: Planning computed tomography with metal skin marker. B: Biopsy needle positioned within the lesion. All the CT-guided bone biopsies were performed under general anesthesia. The skin and adjacent soft tissues were also locally anesthetized with lidocaine or ropivacaine in order to optimize the post-procedural analgesia. The specimens were collected with 8 to 10 gauge needles and sent to the department of pathological anatomy in a jar with formaldehyde. All the specimens were analyzed by oncologic pathologists. Information about the follow-up of these patients during at least six months after the procedure was researched in the cases where the histological results were benign or indeterminate. A standard data form was filled out for all the patients included in the study, with demographic data (sex and age), previous history of malignant neoplasm, data related to the lesion, to the procedure and to the histological result. Lesion data included the affected bone, lesion size, aspect at CT (lytic, sclerotic or mixed), and diagnostic suspicion. Data regarding the procedure included type of needle utilized, presence of complications and if another imaging method (MRI or PET/CT) was utilized as guidance in the procedure. Data regarding the histological results included information on the specimen appropriateness and final diagnosis. Benign lesions were followed-up for diagnostic confirmation. All the data were stored in a databank for the purpose of statistical analysis utilizing the software SPSS 20.0. Descriptive analysis was performed to calculate simple and relative frequencies of the variables. The Student's t test (or non-parametric Mann-Whitney test, as indicated) was utilized for comparison of scalar variables between groups. In cases of three or more groups, the variance analysis (Anova) or the non-parametric Kruskal-Wallis test was utilized. For the categorical variables the tables 2 × 2 and 2 × 3 were utilized, with evaluation of the statistical significance by the chi-squared Pearson calculator with Yates correction or exact Fisher test, as indicated. Results with type I error probability < 5% (p < 0.05) were considered as statistically significant. RESULTS In the study period 186 CT-guided bone biopsies were performed. Most patients were women (57%) and the mean age was 53.0 ± 16.4 years (ranging between 3 and 83 years). In 47 procedures (24.4%) the diagnostic suspicion was of primary bone lesion, and in 139 (74.6%) there was a suspicion of secondary lesion (metastasis) from other known site - among others the most common were breast (32.1%) and prostate (11.8%). In frequency order, the most commonly involved bones were the following: vertebral column (36.0%), hip bones (32.8%), long bones (18.3%), sternum (4.8%), costal arches (4.3%) and others (3.7%). Mean lesion size was 3.1 ± 1.9 cm, ranging from 0.6 to 9.8 cm. In 18 cases (9.7%) the lesion could not be identified at CT, and the biopsy was guided by MRI findings in 8 cases (4.3%) and by PET/CT in 10 cases (5.4%). In such cases, the MRI or PET/CT images were evaluated side by side with the non contrast-enhanced CT images before the procedure to identify the lesion or area of interest to be biopsied. In the other 168 cases (90.3%) the lesion was identified at CT, being characterized as lytic lesion in 49.4%, blastic lesion in 35.1%, and mixed in 15.5% of cases. In most patients (83.1%) the biopsy was performed with an 8-gauge needle. A 10-gauge needle was utilized in 16.9% of the procedures. Severe complications were observed in only three cases (1.6%), including one bone fracture, one patient who presented paresthesia with functional compromise, and one procedure with needle breakage requiring surgical removal. In only three cases (1.6%) the collected specimen was considered inappropriate for diagnosis, including two lytic lesions in the vertebral column and one lytic lesion in the femur. In one of such cases no information about follow-up was available, and in the other two cases no lesion progression was observed at follow-up. Amongst the 183 procedures with appropriate specimens (98.4%), the pathological diagnosis was normal bone tissue/absence of malignancy in 85 cases (45.7%), benign primary bone tumor in 13 (7.0%), malignant primary bone tumor in 9 (4.8%), and metastasis in 76 cases (40.9%). Amongst the 85 patients whose histological diagnosis of the biopsy specimen was normal bone tissue/absence of malignancy, 64 (75.3%) were followed-up and the following events were observed: 6 (6.4%) patients were submitted to open surgery (4 confirmed the diagnosis and 2 were characterized as primary bone tumors - Langerhans cell histiocytosis and non-Hodgkin's lymphoma); 2 (3.1%) were treated as infectious process (osteomyelitis); 49 (76.5%) did not present any alteration at follow-up, and were considered as benign lesions; and 7 (10.9%) presented lesion progression at follow-up, being considered as malignant. Malignant results were most frequently observed in the patients under suspicion of secondary lesion with history of a known malignant neoplasia (Table 1) and at PET/CT-guided procedures (Table 2). No correlation was observed between rate of malignancy at biopsy, size and appearance of the lesion at CT. 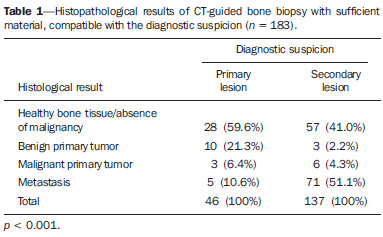 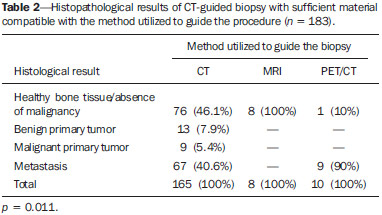 DISCUSSION Imaging methods play a fundamental role in the screening, detection and characterization of bone lesions, besides providing guidance in percutaneous biopsies of lesions suspicious for malignancy. The distribution of the different diagnosis (metastases, primary tumors, benign diseases and others) is quite variable among studies in the literature(23-26). The main indication for biopsy is the investigation of a suspicious metastatic lesion(27). Even in patients with suspicious lesions and history of malignant primary neoplasm, whose chance of a diagnosis different from metastasis is low, the confirmation of the diagnosis is required because it will influence the management and prognosis of these patients. The results of the present study have demonstrated that CT-guided percutaneous biopsy of bone lesions produces a high percentage of specimens sufficient for histological analysis, allowing for a correct diagnosis in the greatest majority of cases. The rates of diagnostic definition and accuracy reported in the literature range from 69% to 96%(7,11-13,18-2,28-34). There are several potential causes for the unsuccess of CT-guided biopsies, including failure in performing biopsy of the target lesion, failure in collecting material sufficient for analysis, impossibility of making a definite diagnosis based on nonspecific histological characteristics, presence of necrosis or imaging artifacts, and lack of confidence in results, requiring a new collection of specimen. Failure in performing biopsy of the target lesion or in obtaining material sufficient for analysis might result from technical factors such as difficulty in approaching the lesion. Such a failure is suggested as the cause of lower rates of success in biopsies in the vertebral column(12,21,29), lesions where a soft part component is not present(12,18), and sclerotic lesions(22,28). Wu et al.(22) have demonstrated a higher rate of success with a greater number of collected specimens, and have proposed the obtention of three specimens for bone lesions as the ideal number. Aiming at obtaining a better rate of success, interventional radiologists should establish objective criteria at the moment they select the biopsy site. Some basic strategies include establish the apparently more aggressive portion of the lesion as the main target, avoiding areas of necrosis. The guidance of the procedure with other imaging methods may also be extremely useful, for example, selecting as a target those areas with greater metabolic activity at PET/CT images (Figure 2). In the present study, the histological results were malignant in almost all PET/CT-guided procedures. Some authors have already demonstrated that PET/CT-guided biopsies of bone lesions and other organs provide a high percentage of appropriate specimens as well of malignant results(35,36). On the other hand, in the present study, all the lesions identified at MRI which did not present correspondence at CT had benign biopsy results, suggesting that one should be careful about indicating a biopsy in such cases. Despite the high sensitivity of MRI for the diagnosis of bone metastases, its specificity is variable and might be increased by means of an appropriate analysis of the several sequences of the study, including contrast-enhanced and diffusion-weighted images(37). 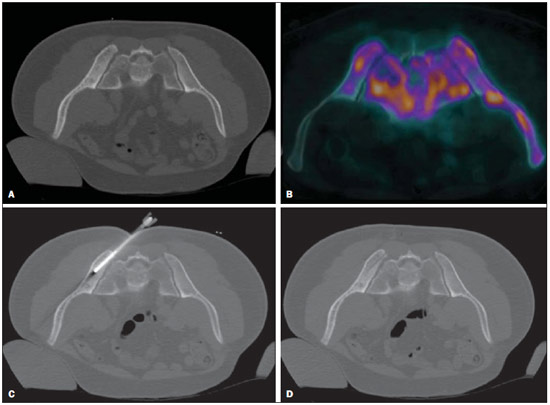 Figure 2. CT-guided and PET/CT-guided percutaneous bone biopsy proved infiltration by non-Hodgkin lymphoma of the iliac bone. A: Planning pelvic CT with metal skin marker demonstrates subtle and diffuse alteration of texture in the left iliac bone. B: PET/CT identifies areas of major metabolic activity, allowing for appropriate selection of the target lesion. C: Needle positioned within the target lesion. D: Post-biopsy follow-up with no immediate complication. The rate o complications observed in the present study was low and compatible with data in the literature, corroborating the fact that percutaneous biopsy of bones lesions is a safe procedure. Rimondi et al. have described 22 complications (1.1%) in 2,027 CT-guided biopsies of lesions in the musculoskeletal system - 18 cases of transient lower limbs paresthesia, 3 hematomas in the psoas muscle, and 1 retroperitoneal hematoma(38). Finally, CT-guided percutaneous biopsy is a safe and effective method for the diagnosis of suspicious bone lesions, with less morbidity and lower cost than open bone biopsy. REFERENCES 1. Chojniak R, Pinto PNV, Tyng CJ, et al. Computed tomographyguided transthoracic needle biopsy of pulmonary nodules. Radiol Bras. 2011;44:315-20. 2. Chojniak R, Grigio HR, Bitencourt AGV, et al. Percutaneous computed tomography-guided core needle biopsy of soft tissue tumors: results and correlation with surgical specimen analysis. Radiol Bras. 2012;45:259-62. 3. Ceratti S, Giannini P, Souza RAS, et al. Ultrasound-guided fineneedle aspiration of thyroid nodules: assessment of the ideal number of punctures. Radiol Bras. 2012;45:145-8. 4. Guimarães MD, Fonte AC, Andrade MQ, et al. Computed tomography-guided core-needle biopsy of lung lesions: an oncology center experience. Radiol Bras. 2011;44:75-80. 5. Queiroz HMC, Costa FA, Campos Jr MM, et al. Arterial embolization in the treatment of hemobilia after hepatic trauma: a case report. Radiol Bras. 2012;45:63-4. 6. Novero ER, Metzger PB, Obregon J, et al. Endovascular treatment of thoracic aortic diseases: a single center result analysis. Radiol Bras. 2012;45:251-8. 7. Altuntas AO, Slavin J, Smith PJ, et al. Accuracy of computed tomography guided core needle biopsy of musculoskeletal tumours. ANZ J Surg. 2005;75:187-91. 8. Jelinek JS, Murphey MD, Welker JA, et al. Diagnosis of primary bone tumors with image-guided percutaneous biopsy: experience with 110 tumors. Radiology. 2002;223:731-7. 9. Leffler SG, Chew FS. CT-guided percutaneous biopsy of sclerotic bone lesions: diagnostic yield and accuracy. AJR Am J Roentgenol. 1999;172:1389-92. 10. Ashford RU, McCarthy SW, Scolyer RA, et al. Surgical biopsy with intraoperative frozen section. An accurate and cost-effective method for diagnosis of musculoskeletal sarcomas. J Bone Joint Surg Br. 2006;88:1207-11. 11. Fraser-Hill MA, Renfrew DL, Hilsenrath PE. Percutaneous needle biopsy of musculoskeletal lesions. 2. Cost-effectiveness. AJR Am J Roentgenol. 1992;158:813-8. 12. Hau A, Kim I, Kattapuram S, et al. Accuracy of CT-guided biopsies in 359 patients with musculoskeletal lesions. Skeletal Radiol. 2002;31:349-53. 13. Welker JA, Henshaw RM, Jelinek J, et al. The percutaneous needle biopsy is safe and recommended in the diagnosis of musculoskeletal masses. Cancer. 2000;89:2677-86. 14. Espinosa LA, Jamadar DA, Jacobson JA, et al. CT-guided biopsy of bone: a radiologist's perspective. AJR Am J Roentgenol. 2008;190:W283-9. 15. deSantos LA, Lukeman JM, Wallace S, et al. Percutaneous needle biopsy of bone in the cancer patient. AJR Am J Roentgenol. 1978;130:641-9. 16. Krause ND, Haddad ZK, Winalski CS, et al. Musculoskeletal biopsies using computed tomography fluoroscopy. J Comput Assist Tomogr. 2008;32:458-62. 17. Omura MC, Motamedi K, UyBico S, et al. Revisiting CT-guided percutaneous core needle biopsy of musculoskeletal lesions: contributors to biopsy success. AJR Am J Roentgenol. 2011;197:457-61. 18. Datir A, Pechon P, Saifuddin A. Imaging-guided percutaneous biopsy of pathologic fractures: a retrospective analysis of 129 cases. AJR Am J Roentgenol. 2009;193:504-8. 19. Harish S, Hughes RJ, Saifuddin A, et al. Image-guided percutaneous biopsy of intramedullary lytic bone lesions: utility of aspirated blood clots. Eur Radiol. 2006;16:2120-5. 20. Ng CS, Salisbury JR, Darby AJ, et al. Radiologically guided bone biopsy: results of 502 biopsies. Cardiovasc Intervent Radiol. 1998;21:122-8. 21. Puri A, Shingade VU, Agarwal MG, et al. CT-guided percutaneous core needle biopsy in deep seated musculoskeletal lesions: a prospective study of 128 cases. Skeletal Radiol. 2006;35:138-43. 22. Wu JS, Goldsmith JD, Horwich PJ, et al. Bone and soft-tissue lesions: what factors affect diagnostic yield of image-guided coreneedle biopsy? Radiology. 2008;248:962-70. 23. Logan PM, Connell DG, O'Connell JX, et al. Image-guided percutaneous biopsy of musculoskeletal tumors: an algorithm for selection of specific biopsy techniques. AJR Am J Roentgenol. 1996;166:137-41. 24. Yao L, Nelson SD, Seeger LL, et al. Primary musculoskeletal neoplasms: effectiveness of core-needle biopsy. Radiology. 1999;212:682-6. 25. Wedin R, Bauer HC, Skoog L, et al. Cytological diagnosis of skeletal lesions. Fine-needle aspiration biopsy in 110 tumours. J Bone Joint Surg Br. 2000;82:673-8. 26. Fraser-Hill MA, Renfrew DL. Percutaneous needle biopsy of musculoskeletal lesions. 1. Effective accuracy and diagnostic utility. AJR Am J Roentgenol. 1992;158:809-12. 27. Vieilleard MH, Boutry N, Chastanet P, et al. Contribution of percutaneous biopsy to definite diagnosis in patients with suspected bone tumor. Joint Bone Spine. 2005;72:53-60. 28. Ayala AG, Zornosa J. Primary bone tumors: percutaneous needle biopsy. Radiologic-pathologic study of 222 biopsies. Radiology. 1983;149:675-9. 29. Dupuy DE, Rosenberg AE, Punyaratabandhu T, et al. Accuracy of CT-guided needle biopsy of musculoskeletal neoplasms. AJR Am J Roentgenol. 1998;171:759-62. 30. Issakov J, Flusser G, Kollender Y, et al. Computed tomographyguided core needle biopsy for bone and soft tissue tumors. Isr Med Assoc J. 2003;5:28-30. 31. Mitsuyoshi G, Naito N, Kawai A, et al. Accurate diagnosis of musculoskeletal lesions by core needle biopsy. J Surg Oncol. 2006;94:21-7. 32. Skrzynski MC, Biermann JS, Montag A, et al. Diagnostic accuracy and charge-savings of outpatient core needle biopsy compared with open biopsy of musculoskeletal tumors. J Bone Joint Surg Am. 1996;78:644-9. 33. Tsukushi S, Katagiri H, Nakashima H, et al. Application and utility of computed tomography-guided needle biopsy with musculoskeletal lesions. J Orthop Sci. 2004;9:122-5. 34. Tsukushi S, Nishida Y, Yamada Y, et al. CT-guided needle biopsy for musculoskeletal lesions. Arch Orthop Trauma Surg. 2009;130:699-703. 35. Werner MK, Aschoff P, Reimold M, et al. FDG-PET/CT-guided biopsy of bone metastases sets a new course in patient management after extensive imaging and multiple futile biopsies. Br J Radiol. 2011;84:e65-7. 36. Bitencourt AG, Tyng CJ, Pinto PN, et al. Percutaneous biopsy based on PET/CT findings in cancer patients: technique, indications, and results. Clin Nucl Med. 2012;37:e95-7. 37. Vanel D. MRI of bone metastases: the choice of the sequence. Cancer Imaging. 2004;4:30-5. 38. Rimondi E, Rossi G, Bartalena T, et al. Percutaneous CT-guided biopsy of the musculoskeletal system: results of 2027 cases. Eur J Radiol. 2011;77:34-42. 1. MD, Resident at Imaging Department - A.C.Camargo Cancer Center, São Paulo, SP, Brazil 2. Master and Fellow PhD degree, Titular of Imaging Department - A.C.Camargo Cancer Center, São Paulo, SP, Brazil 3. PhD, Titular of Imaging Department - A.C.Camargo Cancer Center, São Paulo, SP, Brazil 4. Master, Fellow of the Interventional Radiology Department - A.C.Camargo Cancer Center, São Paulo, SP, Brazil 5. Titular of the Interventional Radiology Department - A.C.Camargo Cancer Center, São Paulo, SP, Brazil 6. PhD, Titular of Pelvic Surgery Department - A.C.Camargo Cancer Center, São Paulo, SP, Brazil 7. PhD, Director, Imaging Department - A.C.Camargo Cancer Center, São Paulo, SP, Brazil. Mailing Address: Dr. Macello Jose Sampaio Maciel Rua Professor Antonio Prudente, 211, Liberdade São Paulo, SP, Brazil, 01509-010 E-mail: macellomaciel@me.com Received October 16, 2013. Accepted after revision March 14, 2014. Study developed at A.C.Camargo Cancer Center, São Paulo, SP, Brazil. |
|
Av. Paulista, 37 - 7° andar - Conj. 71 - CEP 01311-902 - São Paulo - SP - Brazil - Phone: (11) 3372-4544 - Fax: (11) 3372-4554