Radiologia Brasileira - Publicação Científica Oficial do Colégio Brasileiro de Radiologia
AMB - Associação Médica Brasileira CNA - Comissão Nacional de Acreditação
 Vol. 47 nº 3 - May / June of 2014
Vol. 47 nº 3 - May / June of 2014
|
REVIEW ARTICLE
|
|
Walled-off pancreatic necrosis and other current concepts in the radiological assessment of acute pancreatitis |
|
|
Autho(rs): Elen Freitas de Cerqueira Cunha1; Manoel de Souza Rocha2; Fábio Payão Pereira3; Roberto Blasbalg4; Ronaldo Hueb Baroni4 |
|
|
Keywords: Pancreatitis; Pseudocyst; Walled-off pancreatic necrosis. |
|
|
Abstract: INTRODUCTION
Acute pancreatitis is an inflammatory condition caused by intracellular activation and inappropriate extravasation of proteolytic enzymes determining destruction of the pancreatic parenchyma and peripancreatic tissues. Biliary lithiasis and alcoholism are the most common etiological factors accounting for 80% of the cases in adults. Less common causes include hypertriglyceridemia, hypercalcemia, drugs, autoimmune diseases, parasitosis, among others(1). Relatively common, acute pancreatitis is one of the most frequent conditions requiring emergency imaging investigation. In Brazil, according to Datasus, in the period between July 2010 and July 2011, there were 25,660 admissions, with a yearly expenditure of R$ 17,493,378.92 and a mortality rate of 5.9%(2). Most patients with acute pancreatitis present with mild disease, which is self-limited and presents favorable evolution with conservative treatment. However, approximately 20-30% of the cases progress to severe disease, with a significant morbimortality(1). The present study is aimed at describing the current radiological concepts in the imaging evaluation of acute pancreatitis, with emphasis on the definition of the condition currently known as "walled-off pancreatic necrosis", with the purpose of standardizing the terminology among specialists involved in the diagnosis and treatment of such patients. NEW CONCEPTS IN THE CLASSIFICATION OF ACUTE PANCREATITIS The Atlanta classification for acute pancreatitis was proposed in 1992(3) in an attempt to standardize the classification of the severity of acute pancreatitis and its complications. Such a classification played a relevant role in such a context, however, over the time it became noticeable that a review was necessary in order to reflect the developments in the understanding of the disease, including a conceptualization of complex fluid collections that may develop from the process of pancreatic necrosis(4-8). Recently, the Acute Pancreatitis Classification Working Group (APCWG) reviewed such a classification(9) and established new concepts related to diagnosis and phases of the disease, and based on this new approach redefined the radiological classification, introducing new concepts regarding local complications of acute pancreatitis. Diagnosis According to APCWG, the diagnosis of acute pancreatitis is defined as two of the following three criteria are met(9): abdominal pain strongly suggestive of acute pancreatitis; increased amylase serum level, at least three times the normal level; characteristic imaging findings. Disease progression over time With respect to the development of the disease over time, two phases of the disease are currently considered in the course of acute pancreatitis(9), as follows: an early phase, where the disease severity is related to the systemic, and a late phase, where the disease may either evolve to resolution (interstitial edematous pancreatitis), stabilization, or present a prolonged evolution related to the necrosis process (necrotizing pancreatitis). Definition of severity and treatment The importance of the definition of severity in acute pancreatitis is directly related to the institution of the therapeutic approach. Patients with mild acute pancreatitis respond well to conservative treatment, while those patients presenting with necrotizing pancreatitis develop organic dysfunction, requiring intensive treatment and, frequently, therapeutic interventions, with a guarded prognosis(1,10). At the first phase of acute pancreatitis, the disease severity is primarily defined by means of clinical and laboratory criteria. At the second phase of acute pancreatitis, the need for treatment is determined with basis on the clinical progression of the disease, and the type of treatment is defined by morphological imaging findings. Thus, the present review emphasizes the utilization of contrast-enhanced computed tomography (CT) criteria to define the therapeutic approach in the second phase of the disease, considering that the morphological changes can provide guidance for the treatment. CT is considered the gold standard in the imaging evaluation of acute pancreatitis, not only for being an effective method but also for being faster and widely available(7,9,11,12). Magnetic resonance imaging (MRI) has diagnostic and prognostic value comparable to those of CT, but it presents some disadvantages in the clinical scenario. The scans are comparably longer, require more cooperation of the patient (immobility for extended periods and apnea) and are more costly. However, MRI is superior to CT in the characterization of pancreatic/peripancreatic fluid collections, and is an alternative to CT in those situations where the utilization of iodinated contrast is contraindicated, in addition to the nonutilization of ionizing radiation(14-16). Also, the MRI cholangiopancreatography is highly sensitive for detecting cholelithiasis, aiding in the selection of those patients that may require endoscopic cholangiopancreatography(10,17). Ultrasonography plays a relevant role in the evaluation of the biliary tract, but it is frequently limited in the visualization of the distal bile duct (because of intestinal gas) and in the evaluation of the pancreas. IMAGING EVALUATION OF ACUTE PANCREATITIS CT protocol The CT scan must be performed within 48-72 hours after the onset of the clinical condition since, in general, necrosis begins within 24-48 hours(18). The imaging evaluation is recommended to confirm the clinical diagnosis, determine the etiology, rule out other causes of pain associated with increased amylase/lipase levels and determine the severity and extent of acute pancreatitis. The imaging evaluation is necessary in severe or dubious cases, and may become dispensable in cases of mild disease with classic clinical presentation(19,20). The CT scan protocol utilized at Instituto de Radiologia - Hospital das Clínicas da Faculdade de Medicina da Universidade de São Paulo includes imaging performed in 64-channel multidetector apparatus with oral administration of water (600 ml) and intravenous injection of nonionic iodinated contrast agent (1.5 ml/kg), with pre- and post-contrast acquisitions in the arterial and parenchymal phases (40 seconds after starting the contrast medium injection), and venous phase (70 seconds after starting the contrast medium injection), at an injection rate of 4 ml/s, and 2.5 mm reconstructions. The follow-up scan protocols are individualized according to the clinical evolution. Acute interstitial edematous pancreatitis (Figures 1 and 2) 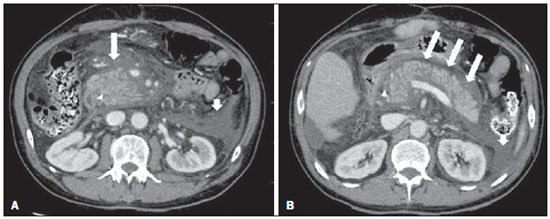 Figure 1. Acute edematous pancreatitis. A,B: Contrast-enhanced axial CT images, venous phase, demonstrating diffuse pancreatic enlargement, densification of the peripancreatic fat planes (long arrows) and acute fluid collections in the left anterior pararenal space and in the left paracolic gutter (short arrows), without areas of parenchymal necrosis. 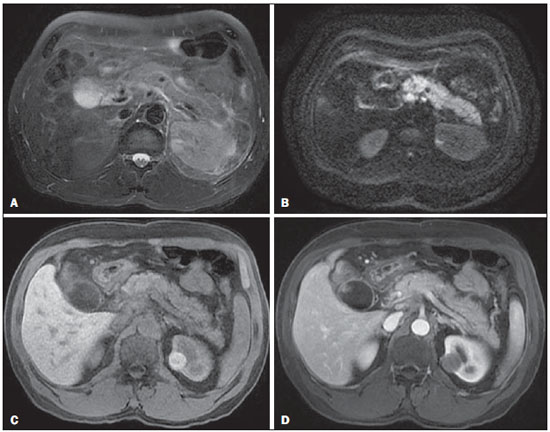 Figure 2. MRI in acute edematous pancreatitis. A: Axial MRI fast-spin echo T2-weighted sequence with fat suppression showing diffuse pancreatic enlargement with increased signal on T2-weighted sequence, loss of the usual glandular pattern and peripancreatic fluid. B: Diffusion-weighted echo planar axial MRI sequence showing water molecules diffusion restriction throughout the entire pancreatic parenchyma. Pre-contrast (C) and contrast-enhanced (D) T1-weighted gradient echo axial MRI sequences with fat suppression showing subtle T1 hyposignal of the pancreatic parenchyma and preserved enhancement, with no area of necrosis. CT may be either normal or demonstrate diffuse or localized pancreatic enlargement, with loss of the usual glandular pattern, but with normal parenchymal enhancement. Frequently, the peripancreatic/retroperitoneal tissues present with subtle inflammatory changes characterized by fat densification and variable amounts of peripancreatic fluid. Interstitial edematous pancreatitis accounts for 80% of the acute pancreatitis cases(1,10). A CT scan performed at an early phase of the disease may demonstrate heterogeneity of the pancreatic parenchyma, which cannot be definitively classified as edematous or necrotizing pancreatitis. In such a context, the case should be classified as undetermined and a follow-up scan within 5-7 days may define the classification of the case(5,9,11,12,21). Necrotizing acute pancreatitis (Figures 3 and 4) 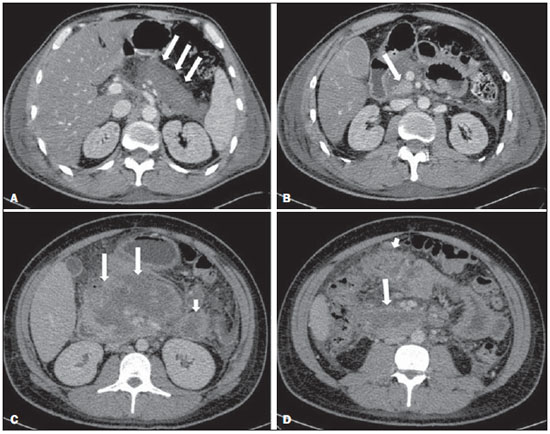 Figure 3. Acute necrotizing pancreatitis. A,B: Contrast-enhanced axial CT images, venous phase. Acute necrotizing pancreatitis in a 52-year-old male patient. Diffuse hypoenhancement of the pancreatic neck, body and tail (arrows on A), compatible with presence of an extensive area of necrosis, with a small area of preserved parenchyma in the uncinate process (arrow on B). C,D: Axial images and contrast-enhanced CT, venous phase. Acute necrotizing pancreatitis in a 35-year- old woman. Extensive areas of pancreatic parenchymal necrosis (long arrows) in association with areas of fat necrosis in the left anterior pararenal space and in the transverse mesocolon (short arrows). 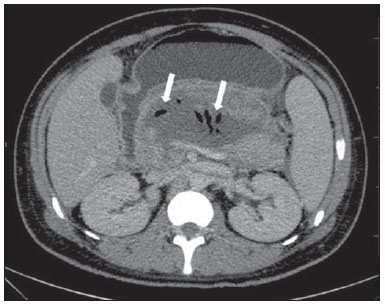 Figure 4. Infected acute necrotizing pancreatitis in a 35-year-old man. Contrastenhanced axial CT image, venous phase showing liquefied area in the pancreatic body, compatible with necrosis, with gas inside (arrows) without an outlined fluidgas level, but intermingled with the fluid, indicating the presence of thick fluid/pus content. In such a context, gas corresponds to the presence of infection. It can be divided into pancreatic parenchyma necrosis, usually concomitant with peripancreatic tissue necrosis, and necrosis restricted to peripancreatic tissues. Each one of the two conditions may or may not be associated with local infectious complications. It occurs in 20-30% of the patients and is characterized by a prolonged course, with high incidence of local complications and a high mortality rate(1,10). Parenchymal necrosis is defined as areas which do not present with enhancement by the contrast agent, and an index scale may be utilized to quantify the percentage of affected parenchyma as follows: < 30%, 30-50%, and > 50%(22). Peripancreatic fat necrosis (steatonecrosis) manifests as peripancreatic tissues densification and heterogeneity. Such a condition may be suggested by the presence of paracolic gutters and mesenteric root thickening, fat densification with involvement of anterior pararenal spaces and, as the disease progresses, by the development of heterogeneous fluid collections containing solid components, as the necrosis features modify over time, sometimes with an initial solid appearance, evolving to a more fluid state. It should be also highlighted that as acute pancreatitis develops, it is more common to observe peripancreatic fat necrosis than pancreatic parenchymal necrosis; moreover, peripancreatic fat necrosis represents a condition with lower morbidity than parenchymal necrosis(23). The definition of infection of pancreatic/peripancreatic necrosis is important due to the clinical implications in the management and prognosis. Secondary bacterial infection occurs in 40-70% of the patients with necrotizing pancreatitis and constitutes the main risk factor for mortality(24). Generally, infection of pancreatic necrosis occurs after the second week of the disease progression(25) and patients with infected necrosis require intensive treatment that may include antibiotic therapy and necrosectomy(26). At CT, the presence of infection may be assumed if extraluminal gas is present in the areas affected by necrosis(18,27), which does not occur in all cases of infected necrosis(7,26) (see Figure 6). The development of a fistula between the pancreatic necrosis and a segment of the gastrointestinal tract is another cause of gas in the pancreas, although it will result in necrosis infection. CT severity index It was proposed by Balthazar et al. in 1990(22), combining the original system of severity evaluation with non-contrast-enhanced CT (based on the evaluation of the presence and number of peripancreatic collections) with the degree of pancreatic necrosis observed at contrast-enhanced CT. Such an index was aimed at improving the early detection of severe presentations of the disease and improving the prognostic value of CT. In terms of morbimortality, a statistically significant correlation was demonstrated between the tomographic classification and clinical staging of the disease(22,28,29). Modified CT severity index It was proposed by Mortele et al. in 2004(30), in an attempt to simplify the original index, considering only the presence or absence of inflammation and peripancreatic collections (with no need for quantifying the number of collections) and excluding the index of necrosis quantification > 50%. On the other hand, the evaluation of extrapancreatic complications was incorporated into the scoring, and demonstrated better correlation with the clinical outcome (hospital stay duration and development of organic failure) than the original index, which was attributed to the inclusion of extrapancreatic changes in the calculation(31). Despite their usefulness in the clinical investigation, the calculations of such indices are not routinely included in reports, and the evaluation of acute pancreatitis severity is always a compilation of the radiological findings with clinical and laboratory results(32,33). Disconnected duct syndrome (Figure 5) 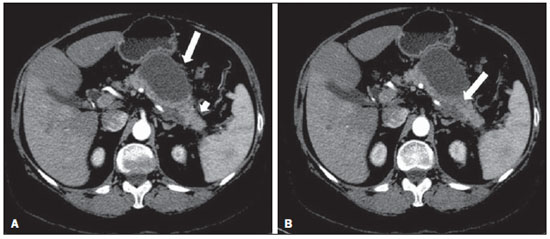 Figure 5. Disconnected duct syndrome. Acute necrotizing pancreatitis with ductal disconnection in a 61-year-old woman. A,B: Contrast-enhanced axial CT images, parenchymal arterial phase showing area of necrosis in the pancreatic body (long arrow on A) affecting a large portion of the parenchymal thickness, pancreatic tail with preserved appearance (short arrow on A). On B, one identifies the main pancreatic duct discharging into the necrotic area (arrow). Disconnected duct syndrome occurs in cases where a parenchymal necrosis area causes the discontinuity of the main pancreatic duct, leaving a portion with preserved drainage downstream of the necrosis and an area of the parenchyma with impaired drainage upstream of the necrosis. Thus, the pancreatic parenchyma upstream of the necrotic area continues producing pancreatic juices, which ends up either accumulating or fistulizing upstream of the necrotic area. Generally, such fluid collections do not spontaneously resolve, requiring surgical drainage, most frequently with pancreaticojejunal diversion, which drains the fluid collection and helps to preserve the function of the pancreatic parenchyma located upstream of the necrotic area(34,35). LOCAL COMPLICATIONS OF ACUTE PANCREATITIS The main recent change in radiological evaluation of acute pancreatitis refers to the utilization of more appropriate terms in the description of fluid collections and areas of necrosis that occur within and around the pancreas. The APCWG proposed that peripancreatic and pancreatic fluid collections were divided into four main categories, according to the elapsed time since the disease onset (more or less of four weeks) and the severity of acute pancreatitis. Thus, both interstitial pancreatitis and necrotizing pancreatitis may course with fluid collections classified as follows. Acute peripancreatic fluid collections (Figure 6) 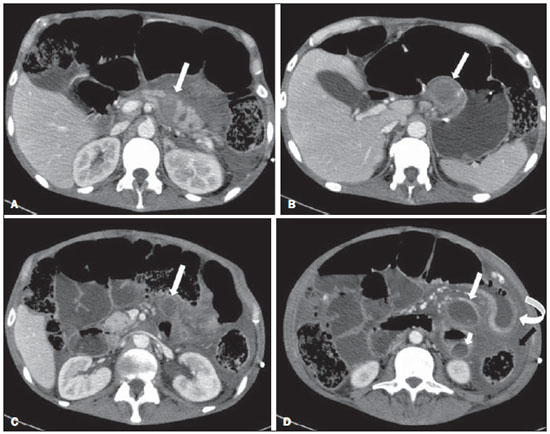 Figure 6. Acute fluid collections in a 52-year-old male patient during the second week of acute necrotizing pancreatitis. A,B,C,D: Contrast-enhanced axial CT images, venous phase showing hypoenhancement of the pancreatic body (arrow on A), compatible with presence of an area of necrosis contiguous with hyperattenuating fluid collection (probable hematic content) in the epiploic retrocavity (arrow on B). Other fluid collections are identified between bowel loops in the peritoneal cavity (long arrows on C, D), in the left anterior pararenal space (short arrow on D), as well as reactive parietal thickening of small loops in the left flank (curved arrow on D) and ascites (black arrow on D). Such fluid collections occur in the first four weeks after symptoms onset, do not have solid components and result from pancreatic or peripancreatic inflammation without necrosis(9). They occur in the peripancreatic spaces, have no defined walls and are frequently located in the epiploic retrocavity and in the anterior pararenal spaces. Generally, such fluid collections result from ductal rupture, but may also result from fluid transudation/edema, without the presence of ductal communication. In most cases, such fluid collections remain sterile and are spontaneously reabsorbed within the first weeks of the acute pancreatitis episode(5,8,9,11,12,15). Pseudocyst (Figures 7, 8 and 9) 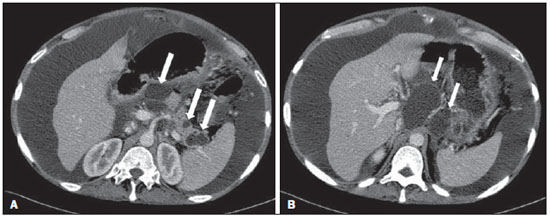 Figure 7. Acute edematous pancreatitis with pseudocysts. A,B: Contrast-enhanced axial CT images, venous phase showing some pseudocysts compressing the pancreatic parenchyma, and others in the epiploic retrocavity (arrows). 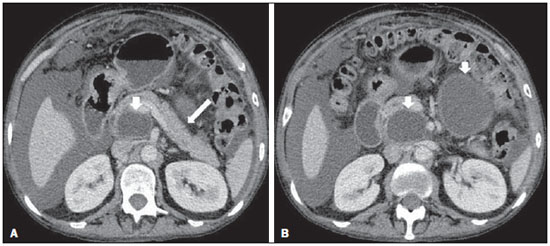 Figure 8. Acute edematous pancreatitis with pseudocysts. A,B: Contrast-enhanced axial CT images, venous phase showing preserved enhancement of the pancreatic parenchyma (long arrow on A), pseudocyst posteriorly to the cephalic segment, uncinate process and in the mesenterium (short arrows on A,B). 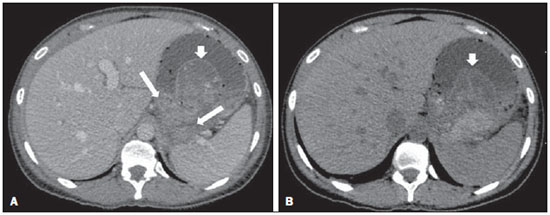 Figure 9. Pseudocyst in acute pancreatitis. A,B: Contrast-enhanced axial CT image, venous phase showing acute inflammatory changes in the pancreatic tail (long arrows on A) and pseudocyst with spontaneously hyperattenuating hematic content (short arrows on A,B) extending toward the left subphrenic space and partially restrained by the gastric wall (short arrows on A,B). It is defined as a round-shaped or ovoid, circumscribed, homogeneous fluid collection with amylase-rich contents, which develops late in the course of acute pancreatitis, about four weeks after the initial event, with no sign of any solid component/tissue necrosis inside. It is surrounded by a granulation tissue capsule with no epithelial lining, in intrapancreatic or extrapancreatic locations(36). Pseudocysts consist in the natural development of peripancreatic fluid collections that persist for more than four weeks, and occur in 10-20% of the patients(22). In the authors' experience, following the criterion of absence of solid contents, the term pseudocyst starts being utilized for the majority of cases with extrapancreatic circumscribed fluid collections. The visualization of pseudocysts' walls (capsule) at diagnostic images is variable and depends on the thickness of such a wall; but one should always observe the circumscribed feature in order to consider a fluid collection as a pseudocyst. The pseudocysts may regress spontaneously or develop with complications such as bleeding (Figure 9) and infection(18). The term pancreatic abscess adopted by the Atlanta classification is no longer utilized and should be replaced by infected pseudocyst, according to APCWG. Post-necrotic pancreatic and peripancreatic changes (Figure 10) 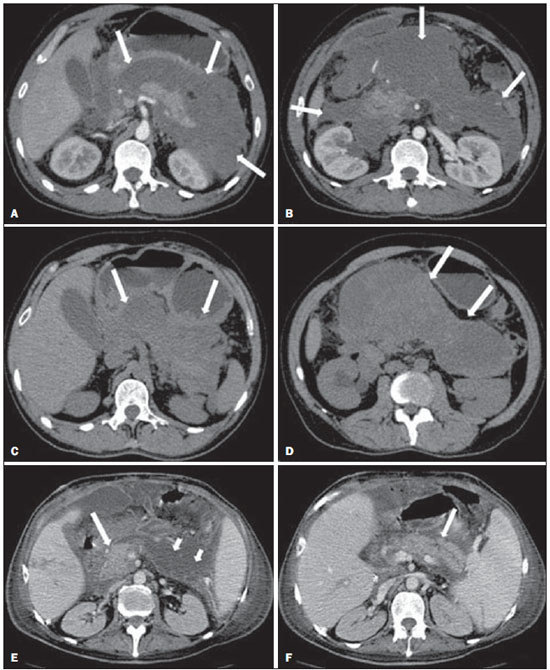 Figure 10. Post-necrotic pancreatic and peripancreatic changes. A,B: Contrast-enhanced axial CT images, venous phase showing extensive areas of peripancreatic fat necrosis (arrows). C,D: Non-contrast-enhanced CT after eight weeks, such areas become more delimited with a liquefied appearance, characterizing postnecrotic pancreatic and peripancreatic changes (arrows). D,E: A 37-year-old patient with acute necrotizing pancreatitis restricted to peripancreatic tissues. Contrast- enhanced axial CT images, venous phase show preserved pancreatic parenchymal enhancement (long arrows on D,E), with extensive areas of peripancreatic fat necrosis (short arrows on D). The patient presented with a septic condition and was submitted to necrosectomy. Purulent material was identified in those areas. Such changes result from a combination of extravasation of pancreatic enzymes, inflammatory exudates, hemorrhage and necrotic residues of parenchyma and/or peripancreatic tissues, including areas of steatonecrosis. Initially, such changes have a solid appearance, becoming liquefied over the course of the disease, generally between two to six weeks from the onset of the disease. As those necrosis areas organize, they become better delimited by a thick wall of granulation tissue, in a process similar to the development of a pseudocyst(5,7,9,11). It may be difficult to differentiate such post-necrotic changes from acute peripancreatic fluid collections, particularly at the first week in the course of the disease; but the follow-up evaluations in general differentiate such two conditions. Walled-off pancreatic necrosis Such a morphological change occurs late in the course of acute necrotizing pancreatitis (approximately four weeks after onset) and consists of a circumscribed area containing fluid and necrotic pancreatic debris replacing part of the pancreatic parenchyma, originating from a necrosis area. Such an entity was not included in the original Atlanta classification and for that reason it is not yet well known. The very translation of the term originally proposed in the English language may initially lead to controversy, however, the radiological concept of a delimited change is well demonstrated by the term "walled-off pancreatic necrosis", that comes from the expression "to wall-off"- meaning to build a wall around a certain location(37) (Figures 11 and 12). 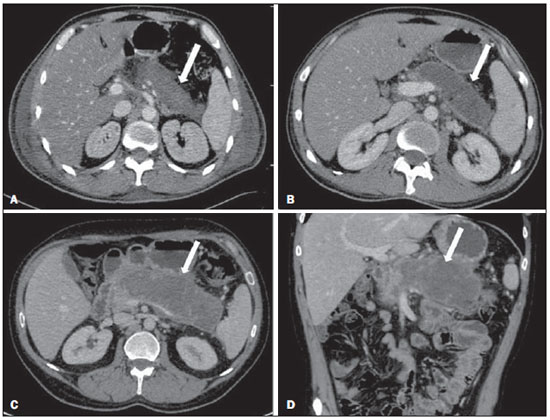 Figure 11. Walled-off pancreatic necrosis. A,B: Contrast-enhanced axial CT images, venous phase. Development of acute necrotizing pancreatitis in a 45-year-old male patient. A: Extensive necrosis of the pancreatic body and tail with ill-defined limits and solid appearance (arrow). B: After two weeks, the delimitation of the necrotic area with a liquefied appearance can already be observed with necrotic debris inside (arrow). C,D: Contrast-enhanced axial (C) and coronal (D) CT images, venous phase. A 42-year-old male patient with circumscribed parenchymal necrosis replacing the pancreatic body and tail (arrows) after three weeks from the onset of acute necrotizing pancreatitis. 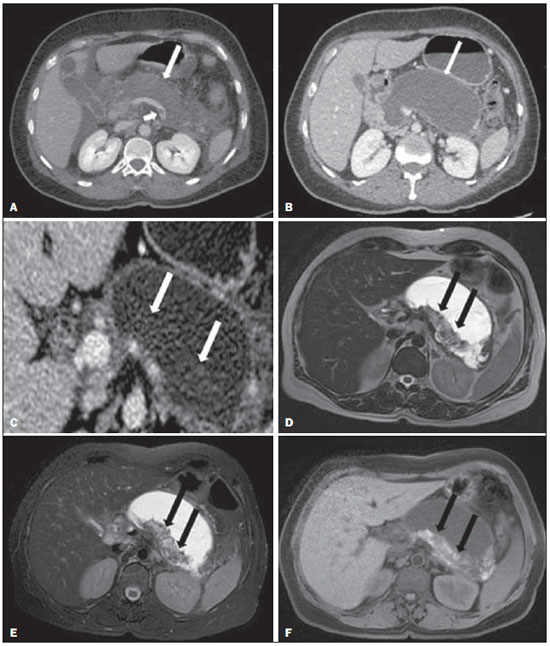 Figure 12. Walled-off pancreatic necrosis. A,B,C: Contrast-enhanced axial CT images, venous phase. Extensive necrosis of the pancreatic body and tail, with peripancreatic inflammatory changes (long arrow on A). Also, a thrombus is identified within the splenic vein (short arrow on A). After approximately one month, an area of walled-off pancreatic necrosis is identified (arrow on B), which should not be confused with pseudocyst. As the same image is evaluated with a narrower window, it is possible to identify the presence of necrotic debris without enhancement within such walled-off pancreatic necrosis (arrows on C). D,E,F: Axial MRI T2-weighted fast spin echo images with (D) and without (E) fat suppression, and non-contrast-enhanced T1-weighted, gradient echo (F) showing necrotic debris of the pancreatic parenchyma deposited in the posterior portion of the collection (arrows on E,F), with signal hyperintensity on T1-weighted sequences, indicating the presence of hemorrhagic component (arrows on E). The Portuguese term proposed in the present study brings to the national literature the concept already consolidated in review articles published in international journals, and the authors believe that the Portuguese term necrose pancreática delimitada can be applied in radiological reports in those situations that fit the above presented description. It is not uncommon that the presence of areas of walledoff pancreatic necrosis be still reported as pseudocysts, but the two entities differ by the fact that walled-off pancreatic necrosis replaces part of the pancreatic parenchyma and presents with thick contents (pancreatic necrotic debris), while pseudocysts do not contain necrotic debris and most commonly develop in the peripancreatic spaces. The characterization of necrotic debris in cases of walled-off pancreatic necrosis is not always simple at CT, but it may be done by analyzing the images with an appropriate ("narrower") window. MRI can more easily demonstrate the necrotic debris(38)(Figure 12). The differentiation is clinically relevant, as such conditions may have different prognosis and require different therapeutic strategies. As previously discussed, pseudocysts have a better prognosis than areas of pancreatic necrosis and, as necessary, they can be more easily drained by endoscopic means than walled-off pancreatic necrosis(7-9,11,39). CONCLUSION Imaging methods still play a fundamental role in the initial evaluation, identification of severe cases, prognosis prediction and in decision making during therapeutic management of patients with acute pancreatitis. The correct differentiation between edematous and necrotizing pancreatitis (both in pancreatic or only peripancreatic presentations) and the appropriate characterization of complex fluid collections related to the pancreatic necrosis process are determining factors for a better management of the patients in what refers to prognosis stratification and definition of the best therapeutic strategy. The definition and standardization of the terms adopted to describe the different changes that may occur in the course of acute pancreatitis are useful to allow for an appropriate dialogue between the different specialists involved in the diagnosis and treatment of such relevant clinical condition. REFERÊNCIAS 1. Vege SS. Clinical manifestations and diagnosis of acute pancreatitis. UpToDate; 2011. [acessado em 20 de setembro de 2011]. Disponível em: https://www.uptodate.com/contents/clinical-manifestations-and-diagnosis-of-acute-pancreatitis. 2. Brasil. Ministério da Saúde. Datasus. Informações de saúde - 2011. [acessado em 25 de setembro de 2011]. Disponível em: http://tabnet.datasus.gov.br/cgi/tabcgi.exe?sih/cnv/niuf.def. 3. Bradley EL 3rd. A clinically based classification system for acute pancreatitis. Summary of the International Symposium on Acute Pancreatitis, Atlanta, Ga, September 11 through 13, 1992. Arch Surg. 1993;128:586-90. 4. Bollen TL, van Santvoort HC, Besselink MG, et al. The Atlanta classification of acute pancreatitis revisited. Br J Surg. 2008;95:6-21. 5. Sheu Y, Furlan A, Almusa O, et al. The revised Atlanta classification for acute pancreatitis: a CT imaging guide for radiologists. Emerg Radiol. 2012;19:237-43. 6. O'Connor OJ, Buckley JM, Maher MM. Imaging of the complications of acute pancreatitis. AJR Am J Roentgenol. 2011;197:W375-81. 7. Morgan DE. Imaging of acute pancreatitis and its complications. Clin Gastroenterol Hepatol. 2008;6:1077-85. 8. Brun A, Agarwal N, Pitchumoni CS. Fluid collections in and around the pancreas in acute pancreatitis. J Clin Gastroenterol. 2011;45:614-25. 9. Acute Pancreatitis Classification Working Group. Revision of the Atlanta classification of acute pancreatitis. [acessado em 20 de agosto de 2011]. Disponível em: http://www.pancreasclub.com/wp-content/uploads/2011/11/Atlanta Classification.pdf. 10. Munsell MA, Buscaglia JM. Acute pancreatitis. J Hosp Med. 2010;5:241-50. 11. Bharwani N, Patel S, Prabhudesai S, et al. Acute pancreatitis: the role of imaging in diagnosis and management. Clin Radiol. 2011;66:164-75. 12. Trout AT, Elsayes KM, Ellis JH, et al. Imaging of acute pancreatitis: prognostic value of computed tomographic findings. J Comput Assist Tomogr. 2010;34:485-95. 13. Arvanitakis M, Koustiani G, Gantzarou A, et al. Staging of severity and prognosis of acute pancreatitis by computed tomography and magnetic resonance imaging - a comparative study. Dig Liver Dis. 2007;39:473-82. 14. O'Connor OJ, McWilliams S, Maher MM. Imaging of acute pancreatitis. AJR Am J Roentgenol. 2011;197:W221-5. 15. Xiao B, Zhang XM. Magnetic resonance imaging for acute pancreatitis. World J Radiol. 2010;2:298-308. 16. Miller FH, Keppke AL, Dalal K, et al. MRI of pancreatitis and its complications: part 1, acute pancreatitis. AJR Am J Roentgenol. 2004;183:1637-44. 17. Makary MA, Duncan MD, Harmon JW, et al. The role of magnetic resonance cholangiography in the management of patients with gallstone pancreatitis. Ann Surg. 2005;241:119-24. 18. Balthazar EJ, Freeny PC, vanSonnenberg E. Imaging and intervention in acute pancreatitis. Radiology. 1994;193:297-306. 19. Working Party of the British Society of Gastroenterology; Association of Surgeons of Great Britain and Ireland; Pancreatic Society of Great Britain and Ireland; Association of Upper GI Surgeons of Great Britain and Ireland. UK guidelines for the management of acute pancreatitis. Gut. 2005;54 Suppl 3:iii1-9. 20. Stevens T, Parsi MA, Walsh RM. Acute pancreatitis: problems in adherence to guidelines. Cleve Clin J Med. 2009;76:697-704. 21. Lenhart DK, Balthazar EJ. MDCT of acute mild (nonnecrotizing) pancreatitis: abdominal complications and fate of fluid collections. AJR Am J Roentgenol. 2008;190:643-9. 22. Balthazar EJ, Robinson DL, Megibow AJ, et al. Acute pancreatitis: value of CT in establishing prognosis. Radiology. 1990;174:331-6. 23. Sakorafas GH, Tsiotos GG, Sarr MG. Extrapancreatic necrotizing pancreatitis with viable pancreas: a previously under-appreciated entity. J Am Coll Surg. 1999;188:643-8. 24. Mazaki T, Ishii Y, Takayama T. Meta-analysis of prophylactic antibiotic use in acute necrotizing pancreatitis. Br J Surg. 2006;93:674-84. 25. Fu CY, Yeh CN, Hsu JT, et al. Timing of mortality in severe acute pancreatitis: experience from 643 patients. World J Gastroenterol. 2007;13:1966-9. 26. Bhansali SK, Shah SC, Desai SB, et al. Infected necrosis complicating acute pancreatitis: experience with 131 cases. Indian J Gastroenterol. 2003;22:7-10. 27. Ranson JH, Balthazar E, Caccavale R, et al. Computed tomography and the prediction of pancreatic abscess in acute pancreatitis. Ann Surg. 1985;201:656-65. 28. Balthazar EJ, Ranson JH, Naidich DP, et al. Acute pancreatitis: prognostic value of CT. Radiology. 1985;156:767-72. 29. Balthazar EJ. Acute pancreatitis: assessment of severity with clinical and CT evaluation. Radiology. 2002;223:603-13. 30. Mortele KJ, Wiesner W, Intriere L, et al. A modified CT severity index for evaluating acute pancreatitis: improved correlation with patient outcome. AJR Am J Roentgenol. 2004;183:1261-5. 31. Mortele KJ, Ip IK, Wu BU, et al. Acute pancreatitis: imaging utilization practices in an urban teaching hospital - analysis of trends with assessment of independent predictors in correlation with patient outcomes. Radiology. 2011;258:174-81. 32. Bollen TL, Singh VK, Maurer R, et al. A comparative evaluation of radiologic and clinical scoring systems in the early prediction of severity in acute pancreatitis. Am J Gastroenterol. 2012;107:612-9. 33. Brisinda G, Vanella S, Crocco A, et al. Severe acute pancreatitis: advances and insights in assessment of severity and management. Eur J Gastroenterol Hepatol. 2011;23:541-51. 34. Sandrasegaran K, Tann M, Jennings SG, et al. Disconnection of the pancreatic duct: an important but overlooked complication of severe acute pancreatitis. Radiographics. 2007;27:1389-400. 35. Varadarajulu S, Noone TC, Tutuian R, et al. Predictors of outcome in pancreatic duct disruption managed by endoscopic transpapillary stent placement. Gastrointest Endosc. 2005;61:568-75. 36. Andrén-Sandberg A, Dervenis C. Pancreatic pseudocysts in the 21st century. Part I: classification, pathophysiology, anatomic considerations and treatment. JOP. 2004;5:8-24. 37. Cambridge Dictionaries Online. [acessado em 28 de setembro de 2011]. Disponível em: http//dictionary.cambridge.org. 38. Morgan DE, Baron TH, Smith JK, et al. Pancreatic fluid collections prior to intervention: evaluation with MR imaging compared with CT and US. Radiology. 1997;203:773-8. 39. Wysocki AP. Walled-off pancreatic necrosis: wishing our pancreatitis nomenclature was correct. World J Gastroenterol. 2010;16:4497-8. 1. MD, Radiologist, Image Memorial/DASA and Diagnoson a+ Medicina Diagnóstica, Salvador, BA, Brasil 2. Private Docent, Associate Professor, Department of Radiology, Faculdade de Medicina da Universidade de São Paulo (FMUSP), São Paulo, SP, Brazil 3. MD, Radiologist, Instituto de Radiologia - Hospital das Clínicas da Faculdade de Medicina da Universidade de São Paulo (InRad/HC-FMUSP), São Paulo, SP, Brasil 4. PhDs, MDs, Radiologists, Instituto de Radiologia - Hospital das Clínicas da Faculdade de Medicina da Universidade de São Paulo (InRad/HC-FMUSP), São Paulo, SP, Brazil Mailing Address: Dr. Manoel de Souza Rocha Avenida Doutor Enéas de Carvalho Aguiar, 255, 3º andar, Cerqueira César São Paulo, SP, Brazil, 05403-900 E-mail: manoelrocha@usp.br Received September 7, 2012. Accepted after revision October 2, 2013. Study developed at Instituto de Radiologia - Hospital das Clínicas da Faculdade de Medicina da Universidade de São Paulo (InRad/HC-FMUSP), São Paulo, SP, Brazil. |
|
Av. Paulista, 37 - 7° andar - Conj. 71 - CEP 01311-902 - São Paulo - SP - Brazil - Phone: (11) 3372-4544 - Fax: (11) 3372-4554