Radiologia Brasileira - Publicação Científica Oficial do Colégio Brasileiro de Radiologia
AMB - Associação Médica Brasileira CNA - Comissão Nacional de Acreditação
 Vol. 47 nº 1 - Jan. /Feb. of 2014
Vol. 47 nº 1 - Jan. /Feb. of 2014
|
WHICH IS YOUR DIAGNOSIS?
|
|
Which is your diagnosis? |
|
|
Autho(rs): Gláucia Zanetti1; Luiz Felipe Nobre2; Alexandre Dias Mançano3; Marcos Duarte Guimarães4; Bruno Hochhegger5; Dante Luiz Escuissato6; Cesar Augusto de Araujo Neto7; Arthur Soares Souza Jr.8; Edson Marchiori1 |
|
|
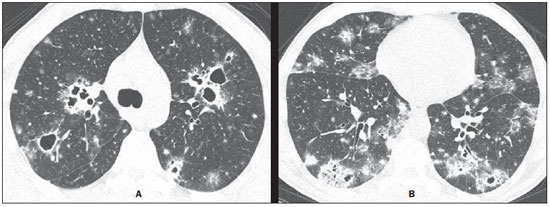
A 49-year-old man with an eight-month history of productive cough, mucopurulent expectoration without blood, progressive dyspnea and asthenia, reported worsening for four months ago, and weight loss (3 kg) in the period. The patient denied fever. Chest radiography demonstrated cavitating pulmonary opacities. Chest high-resolution computed tomography (CT) was performed (Figure 1). The initial routine laboratory tests presented normal results.
Image description Figure 1. Chest high-resolution CT at the level of the carina (A) and lower lobes (B) demonstrates the presence of small nodules, multiple cavitating lesions varying in size in both lungs, besides peripheral, multifocal consolidations and ground glass opacities predominating in the lower lobes. Diagnosis: Pulmonary paracoccidioidomycosis. Mycological analysis of bronchoalveolar lavage was performed and identified the presence of fungus in the direct analysis of the culture medium. COMMENTS Paracoccidioidomycosis (PCM), that is also known as South American blastomycosis, is the most common systemic mycosis in the Latin America and is most frequently found in Brazil, Argentina, Colombia and Venezuela. The most affected endemic areas in the world are located in subtropical regions of Brazil, and the infection is particularly prevalent amongst rural workers. Although most of cases occur in developing countries, recent immigration patterns and the increase in international travels have led to an increasing number of cases of PCM in the United States of America and Europe(1-8). PCM is caused by the dimorphic fungus Paracoccidioides brasiliensis. The disease is acquired by inhalation of infected particles which reach the lungs, causing the primary infection. PCM is characterized by the involvement of lungs, lymph nodes, and chronic progression of mucocutaneous lesions. Lungs constitute the main targets organs of the P. brasiliensis and chronic alterations are responsible for the associated morbidity and mortality. The disease is generally asymptomatic, but may progress to severe lung involvement, leading to progressive cough and dyspnea(1-8). The initial lesion is similar to that of the primary tuberculosis complex, and is either controlled by natural defense mechanisms or progresses to symptomatic disease. Two main clinical presentations are recognized, namely, the acute presentation and a focal/multifocal chronic presentation. The acute presentation of the disease is most common in young patients and involves mainly the reticuloendothelial system, while the chronic presentation is most prevalent in adult male individuals with a predominant pulmonary and mucocutaneous distribution(1-8). Pulmonary findings at chest radiography in patients with chronic PCM are frequently multiple and nonspecific. Such findings have been described as linear reticular opacities, nodules varying in size, ill defined opacities, air-space consolidation and cavitation. In endemic areas, the finding of bilateral and symmetrical opacities in the central regions of the lungs in association with emphysema in the lung bases is suggestive of the disease. In chronic pulmonary PCM, architectural distortion, paracicatricial emphysema and traction bronchiectasis represent common manifestations of fibrosis(9,10). High-resolution CT is frequently performed to investigate pulmonary infections, PMC included. Such imaging method can identify pulmonary abnormalities in patients whose chest radiography presented normal results, and is more sensitive to characterize the pattern and extent of the alterations. The most common CT findings include ground glass opacities, consolidation, small/large nodules, masses, cavitation, interlobular septa thickening, emphysema and fibrotic lesions. Most frequently, parenchymal bands and architectural distortion represent chronic disease. Most of such lesions predominate in the peripheral and posterior regions, involving all the regions of the lungs, with a slight predominance in the middle regions(3-8). PCM is also a relevant cause of reversed halo signal at high-resolution CT and should be taken into consideration in the differential diagnosis(4,11). Recently, cystic masses were described in pulmonary PCM(12). The high-resolution CT findings in patients with acquired immunodeficiency syndrome (AIDS) and PCM are similar to those observed in non-AIDS patients(13). Although the pulmonary involvement is the most common finding in cases of chronic disease, PCM may also present with extrapulmonary alterations such as involvement of the trachea and lymph nodes, pneumothorax and bone lesions. Tracheal PCM and tuberculosis seem to have similar dissemination patterns. The CT findings in patients with tracheal PCM include irregular circumferential thickening of tracheal walls, with submucosal nodules(8,14). Despite the frequent involvement of the lymphatic system, hilar or mediastinal lymph nodes enlargement are uncommon. Abnormality of lymph nodes is reported in only 13% of cases, and pleural effusion is uncommon both in acute and chronic presentations of the disease. Pneumothorax secondary to PCM has been reported, probably caused by rupture of an air-containing space (emphysema, either with or without development of bubbles, or cavitary lesion)(15). Bone involvement in PCM is uncommon and results primarily from hematogenous dissemination of the disease. A review of 173 consecutive cases of acute and chronic PCM has shown bone lesions in only 1.7% of cases(9). In spite of being rare, bone or joint lesions occur most frequently in cases of the juvenile presentation of the disease, particularly in children(16-18). For this reason, the association between pulmonary and bone lesions is extremely rare. The recognition of CT findings associated with pulmonary PCM may be useful for an early diagnosis and institution of the specific treatment, thus reducing the morbidity and mortality associated with the disease. REFERENCES 1. Blotta MH, Mamoni RL, Oliveira SJ, et al. Endemic regions of paracoccidioidomycosis in Brazil: a clinical and epidemiologic study of 584 cases in the southeast region. Am J Trop Med Hyg. 1999;61:390-4. 2. Marchiori E, Moraes HP, Muniz MAS, et al. Paracoccidioidomicose: correlação da tomografia computadorizada de alta resolução com a anatomopatologia. Radiol Bras. 2000;33:333-40. 3. Muniz MAS, Marchiori E, Magnago M, et al. Paracoccidioidomicose pulmonar - aspectos na tomografia computadorizada de alta resolução. Radiol Bras. 2002;35:147-54. 4. Gasparetto EL, Escuissato DL, Davaus T, et al. Reversed halo sign in pulmonary paracoccidioidomycosis. AJR Am J Roentgenol. 2005;184:1932-4. 5. Souza AS Jr, Gasparetto EL, Davaus T, et al. High-resolution CT findings of 77 patients with untreated pulmonary paracoccidioidomycosis. AJR Am J Roentgenol. 2006;187:1248-52. 6. Funari M, Kavakama J, Shikanai-Yasuda MA, et al. Chronic pulmonary paracoccidioidomycosis (South American blastomycosis): high-resolution CT findings in 41 patients. AJR Am J Roentgenol. 1999;173:59-64. 7. Marchiori E, Valiante PM, Mano CM, et al. Paracoccidioidomycosis: high-resolution computed tomography-pathologic correlation. Eur J Radiol. 2011;77:80-4. 8. Barreto MM, Marchiori E, Amorim VB, et al. Thoracic paracoccidioidomycosis: radiographic and CT findings. Radiographics. 2012;32:71-84. 9. Trad HS, Trad CS, Elias Jr J, et al. Radiological review of 173 consecutive cases of paracoccidioidomycosis. Radiol Bras. 2006;39:175-9. 10. Moraes CS, Queiroz-Telles F, Marchiori E, et al. Análise das alterações radiográficas pulmonares durante a terapêutica da paracoccidioidomicose. Radiol Bras. 2011;44:20-8. 11. Freitas Filho M, Gonçalves FG, Basílio MAR, et al. Pulmonary paracoccidioidomycosis and reversed halo sign: a two-case report. Radiol Bras. 2007;40:355-7. 12. Costa AN, Marchiori E, Benard G, et al. Lung cysts in chronic paracoccidioidomycosis. J Bras Pneumol. 2013;39:368-72. 13. Marchiori E, Gasparetto EL, Escuissato DL, et al. Pulmonary paracoccidioidomycosis and AIDS: high-resolution CT findings in five patients. J Comput Assist Tomogr. 2007;31:605-7. 14. Marchiori E, Escuissato DL, Souza AS Jr, et al. Computed tomography findings in patients with tracheal paracoccidioidomycosis. J Comput Assist Tomogr. 2008;32:788-91. 15. Pereira ML, Marchiori E, Zanetti G, et al. Spontaneous pneumothorax as an atypical presentation of pulmonary paracoccidioidomycosis: a case report with emphasis on the imaging findings. Case Rep Med. 2010;2010:961984. 16. Marchiori E, Ferreira EC, Zanetti G, et al. Whole-body magnetic resonance imaging for the evaluation of thoracic involvement in disseminated paracoccidioidomycosis. J Bras Pneumol. 2013;39:248-50. 17. Bayerl JS, Oliveira ARN, Peçanha PM, et al. Osteomyelitis of the wrist in a patient with disseminated paracoccidioidomycosis: a rare presentation. Radiol Bras. 2012;45:238-40. 18. Marchiori E, Dalston M, Zanetti G, et al. Paracoccidioidomycosis: another cause of sternal osteomyelitis. Joint Bone Spine. 2012;79:323-4. 1. Universidade Federal do Rio de Janeiro (UFRJ), Rio de Janeiro, RJ, Brazil 2. Universidade Federal de Santa Catarina (UFSC), Florianópolis, SC, Brazil 3. Radiologia Anchieta – Hospital Anchieta, Taguatinga, DF, Brazil 4. A.C.Camargo Cancer Center, São Paulo, SP, Brazil 5. Santa Casa de Porto Alegre, Porto Alegre, RS, Brazil 6. Universidade Federal do Paraná (UFPR), Curitiba, PR, Brazil 7. Universidade Federal da Bahia (UFBA), Salvador, BA, Brazil 8. Faculdade de Medicina de São José do Rio Preto (Famerp), São José do Rio Preto, SP, Brazil Mailing Address: Dr. Edson Marchiori Rua Thomaz Cameron, 438, Valparaíso Petrópolis, RJ, Brazil, 25685-120 E-mail: edmarchiori@gmail.com Study developed at Universidade Federal do Rio de Janeiro (UFRJ), Rio de Janeiro, RJ, Brazil. |
|
GN1© Copyright 2024 - All rights reserved to Colégio Brasileiro de Radiologia e Diagnóstico por Imagem
Av. Paulista, 37 - 7° andar - Conj. 71 - CEP 01311-902 - São Paulo - SP - Brazil - Phone: (11) 3372-4544 - Fax: (11) 3372-4554
Av. Paulista, 37 - 7° andar - Conj. 71 - CEP 01311-902 - São Paulo - SP - Brazil - Phone: (11) 3372-4544 - Fax: (11) 3372-4554