Radiologia Brasileira - Publicação Científica Oficial do Colégio Brasileiro de Radiologia
AMB - Associação Médica Brasileira CNA - Comissão Nacional de Acreditação
 Vol. 46 nº 6 - Nov. / Dec. of 2013
Vol. 46 nº 6 - Nov. / Dec. of 2013
|
CASE REPORT
|
|
Unilateral pulmonary veins atresia: evaluation by computed tomography |
|
|
Autho(rs): Diego André Eifer1; Felipe Veras Arsego1; Felipe Soares Torres2 |
|
|
Keywords: Pulmonary veins; Unilateral pulmonary veins atresia; Computed tomography. |
|
|
Abstract: INTRODUCTION
Venous abnormalities of the thorax may involve either systemic or pulmonary veins, ranging from incidental findings to components of more complex abnormalities, most frequently congenital heart disease(1). However, complete absence of pulmonary venous drainage into the left atrium is a rare condition and may affect a whole lung, without associated anomalous drainage(2). Atresia may be divided into common, individual or unilateral(3); the latter is a rare condition corresponding to the absence of pulmonary veins in one of the lungs. In the present report, the authors describe the case of an adult patient with unilateral pulmonary veins atresia, with emphasis on computed tomography findings. CASE REPORT A female, 18-year-old patient attended the emergency service with history of hemoptysis. At pulmonary auscultation, the patient presented decreased vesicular murmur in the right lung base, with no abnormality in laboratory tests. Chest radiography did not demonstrate signs of pulmonary infection, but demonstrated right lung and pulmonary artery with reduced dimensions and mediastinal shift to the right (Figure 1). The patient presented history of dyspnea during stress, without recurrent infection. Computed tomography study was performed in a multidetector Philips Brilliance 16 equipment, with 2.0 mm collimation, 1.0 mm thick slices and intravenous non-ionic iodinated contrast agent (80 ml at a rate of 4.5 ml/s) and demonstrated absence of right pulmonary arteries (Figure 2), right pulmonary artery hypoplasia with ipsilateral prominence of bronchial arteries (Figure 3), besides reduction of pulmonary volume at the right lung, allowing the diagnosis. The analysis of the lung parenchyma demonstrated ground glass opacities and peribronchovascular thickening and interlobular septa thickening at the right lung (Figure 4). No other pleuropulmonary or mediastinal changes were identified at computed tomography. Fibrobronchoscopy demonstrated diffuse hypervascularization of the right respiratory tree and mucosal edema with bleeding spots. Scintigraphy was also performed, demonstrating absence of 99mTc-MAA uptake in the right lung. Superselective embolization of hemorrhagic vessels was successfully performed. Currently, the patient remains under follow-up on an outpatient basis, with no new episode of hemoptysis. 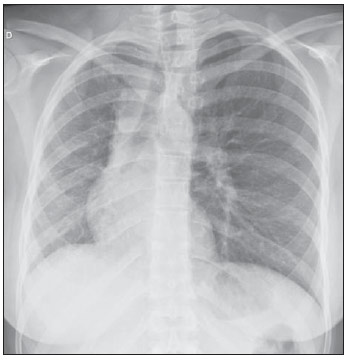 Figure 1. Chest radiography demonstrating right lung with reduced dimensions and ipsilateral mediastinal deviation. 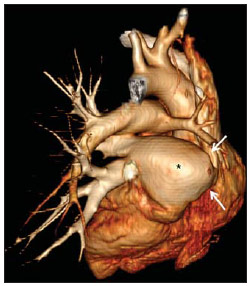 Figure 2. Posterior view of three-dimensional reconstruction of contrast-enhanced chest computed tomography image, with removal of the descending aorta, demonstrating the absence of right pulmonary venous drainage (white arrows) into the left atrium (asterisk). 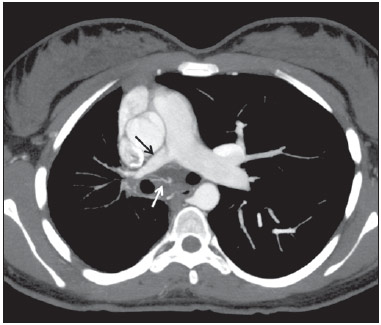 Figure 3. Axial reconstruction of contrast-enhanced chest computed tomography with maximum intensity projection (MIP), demonstrating reduction of right pulmonary artery caliber (black arrow) and prominence of bronchial arteries (white arrow). 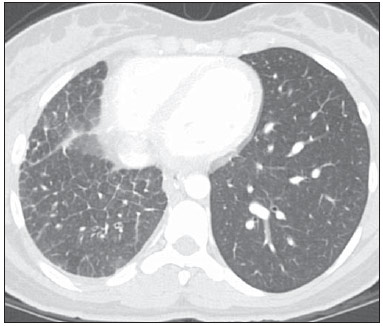 Figure 4. Axial section of chest computed tomography demonstrating interlobular septa thickening and ground glass opacities in the right lower lobe. DISCUSSION Unilateral pulmonary veins atresia is a rare condition, with less than 40 cases reported in the literature until 2010(4). Such a condition is characterized by the absence of pulmonary veins on the affected side and presents high morbimortality(2). Its etiology is unknown, but it is suspected that there may be a congenital failure in the incorporation of the common pulmonary vein into the left atrium, with equal involvement either at right or at left(5). The condition may become clinically noticeable in the childhood or adolescence, with progressive dyspnea caused, at least partially, by pulmonary artery hypertension, recurrent episodes of pneumonia and/or hemoptysis in the affected lung. Cyanosis is not frequently observed in this condition, and one should raise the hypothesis of the presence of a concomitant congenital heart defect, which occurs in 32% to 50% of cases(2,5,6). Physiopathological changes in the affected lung involve hypertrophy and fibrosis of the remaining veins, with fibrosis of the intima and reduction of the vascular lumen(2). Consequently, collateral extrapulmonary vessels develop to partially drain the affected lung. Discontinued (or focal) interstitial fibrosis and interlobular septa thickening secondary to recurrent infections or infarction are observed. Because of inappropriate gaseous exchanges caused by alterations between ventilation and perfusion, there is a progressive reduction in the caliber of the pulmonary artery of the affected lung which, eventually, presents flow reversal toward the contralateral artery. The diagnosis is generally made at the first years of life(5), and the symptoms are extremely variable, ranging from no symptom (7,8) to recurrent pulmonary infections(5,9), hemoptysis(9), and even death(2,4). Death was the outcome in ten of the 25 pediatric cases reported until 2003(2) and also in a recently reported case where the patient presented associated heart defects(10). Since 2011, nine cases have been reported(4,7-12). Out of such cases, four were asymptomatic and the others presented cyanosis(10,12), dyspnea(10-12), hemoptysis(9) or recurrent infections(9). In the present case, the patient had remained asymptomatic for a long period of time. Similarly, Kim et al. have reported two cases of adult patients (23 and 37 years) who were asymptomatic at the diagnosis(4). The time for development of symptoms or pulmonary arterial hypertension seems to be dependent on the balance between the systemic vessels which supply and those vessels which drain the affected lung(2,4), in association with the lymphatic system drainage capacity. In the present case, pulmonary scintigraphy did not demonstrate contrast uptake in the affected lung, indicating a significant decrease in the organ perfusion without detecting of anomalous venous return. Although the CT evaluation of the participation of bronchial veins in the pulmonary drainage is limited, smooth interlobular septal thickening and nodular thickening of interlobular fissures and pleura suggest the presence of a significant lymphatic engorgement. A series of recent studies published in Brazil have highlighted the relevant role of imaging methods in the assessment of the chest(13-17). Chest radiography findings may reveal reduced hemithorax, with ipsilateral mediastinal deviation and parenchymal opacities. Computed tomography demonstrates pulmonary artery with a reduced caliber and absence of pulmonary venous drainage into the left atrium in the affected lung, besides pulmonary alterations such as interlobular septal thickening, peribronchovascular thickening, interstitial fibrosis and ground glass opacities(5,9,18). Ventilation perfusion scintigraphy demonstrates absence of contrast uptake in the affected lung, despite the normal ventilation for the reduced pulmonary volume(6). The therapeutic options include follow-up of patients with few or none symptoms(4,11), selective embolization of systemic collaterals in cases of hemoptysis(5), and pneumonectomy in the presentations with progressive dyspnea, recurrent infections and to prevent pulmonary hypertension without significant left to right shunt(2,4). Although Pourmoghadam et al. indicate surgery as a first therapeutic option(2), Kim et al. understand that such approach may be delayed in adult symptomatic patients provided they are followed-up for development of pulmonary arterial hypertension(4). This was the therapeutic approach adopted in the present case, considering that the superselective embolization of the hemorrhagic vessels was sufficient to control symptoms. Recent publications report four cases where the patients were clinically followed-up, with no complication(4,7,8,11). Two out of such patients underwent follow-up for up to five years(4,8). Surgical repair of anomalous venous return may be performed in cases where there is no significant left to right shunt. But, considering that the diagnosis is many times made after the development of such complication, pneumonectomy of the affected side could reduce the hemodynamic effects of the shunt and reduce the dead space, with reflection on the tolerance to exercises and on the occurrence of repeated infections(2,11). In the present case report, the authors demonstrate the capacity of computed tomography to reveal characteristic findings of unilateral pulmonary veins atresia, reinforcing recent data in the literature indicating the utilization of contrast-enhanced CT as a definitive diagnostic method for this disease and also for other thoracic venous abnormalities(4,9,10,19), reducing the necessity of invasive diagnostic procedures and facilitating the surgical planning (9,18). REFERENCES 1. Demos TC, Posniak HV, Pierce KL, et al. Venous anomalies of the thorax. AJR Am J Roentgenol. 2004;182:1139-50. 2. Pourmoghadam KK, Moore JW, Khan M, et al. Congenital unilateral pulmonary vein atresia: definitive diagnosis and treatment. Pediatr Cardiol. 2003;24:73-9. 3. Lee HN, Kim YT, Cho SS. Individual pulmonary vein atresia in adults: report of two cases. Korean J Radiol. 2011;12:395-9. 4. Kim Y, Yoo IR, Ahn MI, et al. Asymptomatic adults with isolated, unilateral right pulmonary vein atresia: multidetector CT findings. Br J Radiol. 2011;84:e109-13. 5. Heyneman LE, Nolan RL, Harrison JK, et al. Congenital unilateral pulmonary vein atresia: radiologic findings in three adult patients. AJR Am J Roentgenol. 2001;177:681-5. 6. Tissot C, Corbelli R, Aggoun Y, et al. Bronchoscopic diagnosis of asymptomatic unilateral pulmonary vein atresia in an infant. Pediatr Cardiol. 2008;29:976-9. 7. Wang Y, Ma Y, Li B, et al. Unilateral left pulmonary vein atresia: radiologic findings in an adult case. Quant Imaging Med Surg. 2012;2:296. 8. Gasparetto TD, Daltro P, Marchiori E. Imaging findings of an asymptomatic child with pulmonary vein atresia. Pediatr Radiol. 2010;40:1458-9. 9. Dixit R, Kumar J, Chowdhury V, et al. Case report: Isolated unilateral pulmonary vein atresia diagnosed on 128-slice multidetector CT. Indian J Radiol Imaging. 2011;21:253-6. 10. Vergales JE, West SC, Hoyer AW. Pulmonary vein atresia with severe contralateral pulmonary vein stenosis in a child. Pediatr Cardiol. 2012;33:663-5. 11. Savaş Bozbaş Ş, Varan B, Akçay Ş. Right pulmonary venous atresia: a case report and review of literature. Tuberk Toraks. 2012;60:254-7. 12. Kozak MF, Kozak AC, Souza AS, et al. Left pulmonary vein atresia: the contribution of multislice computed tomography. Pediatr Cardiol. 2011;32:108-10. 13. Canellas R, Kanaan D, Martins PHR, et al. Regressão espontânea de proteinose alveolar pulmonar: relato de caso. Radiol Bras. 2012;45:294-6. 14. Chojniak R, Pinto PNV, Ting CJ, et al. Biópsia transtorácica de nódulos e massas pulmonares dirigida por tomografia computadorizada. Radiol Bras. 2011;44:315-20. 15. Koenigkam-Santos M, Barreto ARF, Chagas Neto FA, et al. Tumores neuroendócrinos do pulmão: principais achados radiológicos em uma série de 22 casos com confirmação anatomopatológica. Radiol Bras. 2012;45:191-7. 16. Cerci JJ, Takagaki TY, Trindade E, et al. A tomografia por emissão de pósitrons com 2-[18F]-fluoro-2-desoxi-D-glicose é custo-efetiva em pacientes com câncer de pulmão não pequenas células no Brasil. Radiol Bras. 2012;45:198-204. 17. Bozi LCF, Melo ASA, Marchiori E. Calcificação pulmonar metastática: relato de caso. Radiol Bras. 2012;45:297-9. 18. Mataciunas M, Gumbiene L, Cibiras S, et al. CT angiography of mildly symptomatic, isolated, unilateral right pulmonary vein atresia. Pediatr Radiol. 2009;39:1087-90. 19. Kimura LY, Fernandes GSS, Nobrega KTM, et al. Multidetector-row computed tomography angiography for the diagnosis of anomalous pulmonary venous drainage: an initial experiment. Radiol Bras. 2010;43:347-53. 1. MDs, Residents, Unit of Radiology, Hospital de Clínicas de Porto Alegre (HCPA), Porto Alegre, RS, Brazil 2. MD, Radiologist, Unit of Radiology Hospital de Clínicas de Porto Alegre (HCPA), Program of Post-Graduation in Cardiology - School of Medicine, Universidade Federal do Rio Grande do Sul (UFRGS), Porto Alegre, RS, Brazil Mailing Address: Dr. Felipe S. Torres Serviço de Radiologia, Hospital de Clínicas de Porto Alegre Rua Ramiro Barcelos, 0100-3984, 2350, Santana Porto Alegre, RS, Brazil, 90035-903 E-mail: felipesoarestorres@gmail.com Received September 28, 2012. Accepted after revision April 30, 2013. Study developed in the Unit of Radiology at Hospital de Clínicas de Porto Alegre (HCPA), Porto Alegre, RS, Brazil. |
|
Av. Paulista, 37 - 7° andar - Conj. 71 - CEP 01311-902 - São Paulo - SP - Brazil - Phone: (11) 3372-4544 - Fax: (11) 3372-4554