Radiologia Brasileira - Publicação Científica Oficial do Colégio Brasileiro de Radiologia
AMB - Associação Médica Brasileira CNA - Comissão Nacional de Acreditação
 Vol. 46 nº 4 - July / Aug. of 2013
Vol. 46 nº 4 - July / Aug. of 2013
|
ICONOGRAPHIC ESSAY
|
|
Imaging evaluation of middle ear cholesteatoma: iconographic essay |
|
|
Autho(rs): Ana Flávia Assis de Ávila1; Bruna de Oliveira Melim Aburjeli1; Wanderval Moreira2; Emília Guerra Pinto Coelho Motta3; Marcelo Almeida Ribeiro3; Renata Lopes Furletti Caldeira Diniz3 |
|
|
Keywords: Computed tomography; Middle ear; Cholesteatoma. |
|
|
Abstract: INTRODUCTION
Cholesteatoma is a proliferation of keratinized, stratified squamous epithelium in an anomalous location, generally in the middle ear. It has an osteolytic potential, which may be explained by the presence of collagenase in the lesion periphery, since collagen is the main protein of the bone tissue.(1–4) The term "cholesteatoma" is controversial. According to its literal transcription, the word derives from "cholesterol" and "tumor", but in truth it is an epidermoid cyst. Cholesterol crystals are not observed in the structure of the lesion which also does not present a tumor-like nature(1,2). PATHOGENESIS There are four main theories regarding the origin of acquired cholesteatomas, namely: retraction, epithelial invasion, middle ear epithelium metaplasia, and hyperplasia of basal cells(1–3). The most widely accepted theory suggests that cholesteatomas arise from retraction pockets. If a negative pressure develops in the middle ear (because of auditory tube dysfunction or infection), the tympanic membrane retracts, generally in its weakest part – the pars flaccida. Thus, the keratin (which typically is externally located in relation to the tympanic membrane) undergoes invagination, remaining in an anomalous position. CLASSIFICATION Cholesteatomas may be either congenital or acquired(1–3). Congenital cholesteatomas (2%) originate from embryonic epithelial remains. The patients are born with the tissue in an anomalous position. There is no history of infection, and the tympanic membrane is intact. The lesions tend to occur in the anterior tympanic cavity, proximal to the epitympanum or stapes(1,4). Acquired cholesteatomas are more commonly found (98%), and are related to a chronic inflammatory process in the middle ear. Its etiology is associated with the tympanic membrane anatomy which includes three cell layers, an outer layer in continuity with the mucosa of the external ear conduct, an inner layer in continuity with the mucosa of the middle ear, and a middle layer of fibrous tissue that is present only in the pars tensa(1). Acquired cholesteatomas may occur in the pars flaccida (82%) and pars tensa (18%), most commonly arising in the upper portion of the tympanic membrane pars flaccida, extending towards the Prussak's space, located in the epitympanum and limited by the pars flaccida, by the lateral ligament of the malleus, and by the short process of the malleus (Figure 1)(5). 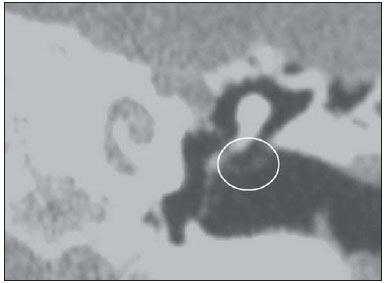 Figure 1. MSCT with coronal multiplanar reconstruction showing Prussak's space delimited by pars flaccida, by the lateral ligament and by the short process of the hammer (circle). Because of its location in the Prussak's space, acquired cholesteatomas of the pars flaccida generally displace the malleus head and the body of the incus medially, causing erosion of the bone spur of Chausse(Figure 2). From the Prussak's space, the mass easily extends itself posteriorly in the superior incudal space towards the posterolateral portion of the attic, and then, through the aditus ad antrum, towards the antrum and the mastoid air cells(4,6). 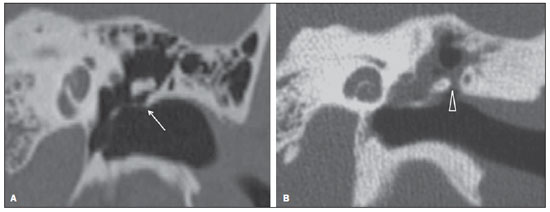 Figure 2. MSCT with coronal multiplanar reconstruction. A: Lateral wall of the attic/intact bone spur of Chausse (arrow). B: Presence of soft-tissue within the middle ear in association with erosion of the lateral wall of the attic/ bone spur of Chausse (arrowhead). Pars tensa cholesteatomas are much less common than pars flaccida cholesteatomas. The medial wall of the epitympanum is most affected, with destruction of ossicles and lateral displacement of the ossicular chain. The lateral wall of the epitympanum and the Prussak's space are spared(4,7). DIAGNOSIS The diagnosis of cholesteatoma is based on clinical evaluation (otoscopy), where retraction of the tympanic membrane with pars flaccida perforation and a whitish mass in the middle ear are observed(7). Multislice computed tomography (MSCT) is considered the imaging method of choice in the evaluation of middle ear cholesteatomas. Magnetic resonance imaging (MRI) has gained importance in the evaluation of complicated cholesteatomas and in the postoperative follow-up of patients to evaluate residual or recurrent cholesteatoma(1,2). Role played by preoperative imaging: evaluation of lesion extent (attic, antrum and mastoid); detection of complications, such as bone lysis and cerebromeningeal complications; and detection of anatomical variations (jugular dehiscence and lateralized sinus). Special attention should be given to the tympanic sinus and to the facial recess, since they are locations whose visualization is difficult during the surgery, and are frequent sites of residual disease. COMPUTED TOMOGRAPHY Preoperative MSCT is a nonspecific method, but is highly suggestive of the disease, with the classical image of a homogeneous, noncalcified, lobulated soft-tissue mass in the attic/antrum, particularly in the Prussak's space and in the epitympanum, presenting mass effect with medial displacement of the ossicular chain, fine structures (lateral attic wall, tympanic tegmen and ossicles) osteolysis, and tympanic membrane retraction (Figure 3). 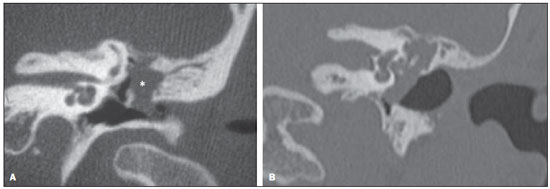 Figure 3. MSCT with coronal multiplanar reconstruction. A: Soft-tissue occupying the Prussak's space and the epitympanum, medially displacing the ossicular chain (asterisk). B: Practically complete obliteration of middle ear by soft-tissue, with ossicular chain demyelination. MSCT is highly accurate to demonstrate the presence of abnormal tissue in the middle ear, with sensitivity ranging between 70% to 96%(5,6), but one of its limitations is that it cannot define if the tissue is inflammatory, fibrotic or cholesteatoma. However, the presence of bone erosion of some structures such as ossicular chain, tympanic tegmen, bone labyrinth and lateral wall of the attic, is strongly indicative of cholesteatoma or chronic otitis media. The differential diagnosis between such two conditions cannot be accurately done with MSCT, since the radiological findings are very similar and many times overlap to each other. In such cases, and particularly in already operated patients, MRI is essential to define the diagnosis. The differential diagnoses of a middle ear mass include cholesteatoma, rhabdomyosarcoma, Langerhans cell histiocytoma, squamous cell carcinoma, metastasis, giant cell tumor and xanthoma(1). MAGNETIC RESONANCE IMAGING MRI increases the specificity as compared with MSCT, particularly in the postoperative follow-up. It is useful to evaluate meningoencephaloceles, to differentiate between fibrosis, inflammatory tissue and disease recurrence. Several MRI protocols have been proposed, and are based principally on delayed post-gadolinium (45 minutes) T1weighted sequences, diffusion-weighted sequences, or on a combination of both techniques(8). As regards diffusion-weighted sequences, distinction should be made between echo-planar sequences (EPI) and non-echo-planar (TSE) sequences. Nonecho-planar diffusion-weighted sequences have a thinner thickness and are less predisposed to susceptibility artifacts than echo-planar sequences. Studies demonstrate that diffusion-weighted sequences are superior to delayed post-gadolinium T1-weighted sequences, and that diffusion-weighted TSE sequences (90% sensitivity and 100% specificity) are superior to diffusion-weighted EPI sequences(8,9). Delayed contrast-enhanced T1weighted sequence (45 minutes) – Cholesteatomas are avascular and is not contrast-enhanced. Fibrosis is poorly vascularized and contrast uptake occurs with delayed acquisitions. On the other hand, inflammatory tissue presents early contrast-enhancement (Figure 4)(2,9). 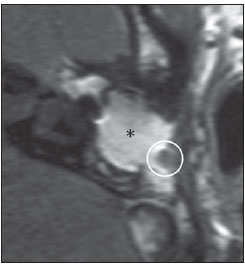 Figure 4. Axial MRI, delayed, contrast-enhanced T1-weighted sequence. Inflammatory tissue/granulation with intense contrast uptake (asterisk). Noncontrast-enhanced recurrent cholesteatoma (circle). Diffusion-weighted sequence – Cholesteatomas present high signal intensity at diffusion-weighted sequences and are differentiated from inflammatory and fibrotic tissues which do not present diffusion restriction (Figures 5 and 6)(2,9). 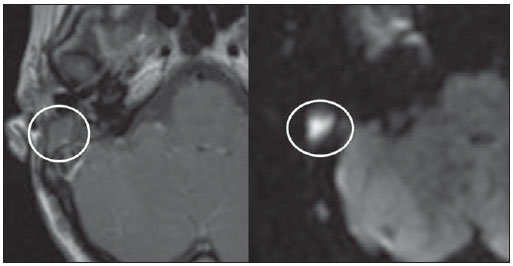 Figure 5. Recurrent cholesteatoma. At left, MRI delayed contrast-enhanced T1-weighted image: absence of contrast uptake. At right, MRI diffusion-weighted EPI: hypersignal compatible with diffusion restriction. 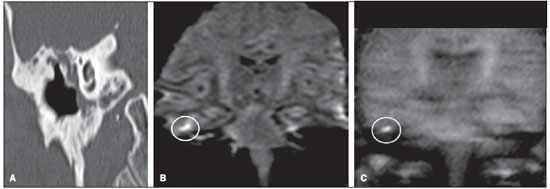 Figure 6. A: MSCT with coronal multiplanar reconstruction: nonspecific soft-tissue within the middle ear. B,C: Restriction on the EPI sequence (B) and TSE sequence (C), compatible with recurrent cholesteatoma (Images kindly provided Dr. Marcelo Garcia). COMPLICATIONS Ossicular chain osteolysis (Figure 7) – It is a frequent complication and leads to transmission deafness. It occurs in 75% of pars flaccida cholesteatomas, and in up to 90% of pars tensa cholesteatomas. Long apophysis of the incus, because of its limited ligament support and poor blood supply, is the most affected ossicular chain segment (87% of cases). The stapes should also be carefully evaluated, since it is compromised in 21% of cases. Amputation of hammer head and body of the incus occurs in very advanced lesions, particularly those occurring in the Prussak's space(4). The comparison with the contralateral ossicles is essential in the diagnosis of very subtle erosions. 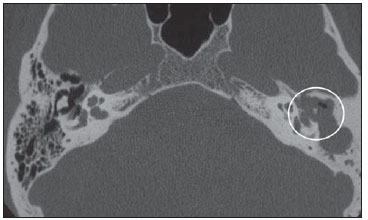 Figure 7. Axial MSCT. Cholesteatomatous lesion in middle ear associated with ossicular chain osteolysis at left (circle). Erosion of the lateral wall of the attic (Figure 2) – The origin of the greatest majority of acquired cholesteatomas in the pars flaccida of the tympanic membrane with extension through the Prussaks's space justifies, as one of the first tomographic findings, the destruction of the bone spur of Chausse located in the junction of the lateral wall of the attic with the wall of the external auditory canal(1). Tympanic tegmen lysis (Figure 8) – Bone dehiscence with risk for development of meningoencephaloceles and epidural invasion by cholesteatoma, increasing the potential for development of meningitis, cerebritis or abscess. MRI is recommended to evaluate such complications. A comparison with the contralateral tympanic tegmen should be carried out, since sometimes the bone thickness is already much reduced. 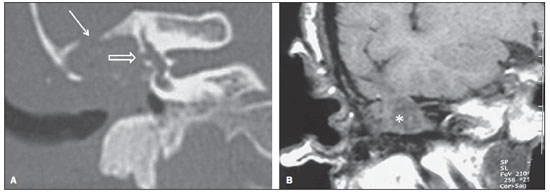 Figure 8. A: MSCT with coronal multiplanar reconstruction: Thinning with bone destruction of the tympanic tegmen (oblique arrow) in association with labyrinth fistula (hollow arrow). B: Coronal MRI: extension of encephalic tissue into the middle ear through tympanic tegmen dehiscence (asterisk). Other diagnoses which may be considered in cases of tympanic tegmen erosion include arachnoid granulations and expansile lesions of the middle cranial fossa with bone involvement. Lysis of facial nerve canal (Figure 9) – The facial nerve canal may be eroded and the function of the nerve can be spared. Facial palsy occurs in approximately 1% to 4% of patients with cholesteatoma. The most common site of facial nerve compression is the tympanic segment, which is located inferiorly to the lateral semicircular canal and above the oval window. The tympanic segment of the facial nerve may be covered by a very thin bone layer or may be an open canal with the nerve exposed to the middle ear(6). 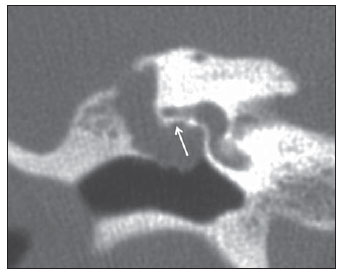 Figure 9. MSCT with coronal multiplanar reconstruction. Bone lysis of the facial nerve tympanic segment (arrow). Labyrinth fistula (Figure 8A) – Potentially severe complication from cholesteatoma, with incidence of 5% to 10%. The lateral semicircular canal is the most compromised region. The diagnosis of fistula can be done as the mass is in direct aposition to the labyrinth lumen. Complications originated from the development of fistulas include neurosensory deafness, dizziness, tinnitus and labyrinthitis. Others – Mastoiditis, either with or without osteitis; extension to the external auditory canal; Bezold abscess (abscess caused by mastoid cortex rupture and extension towards soft tissues); intracranial complications: meningitis, cerebral/cerebellar abscess, subdural empyema and sigmoid sinus thrombophlebitis. Other specific presentations of cholesteatomas – Evacuated cholesteatoma (automastoidectomy): Cholesteatoma evacuation may spontaneously occur through the external auditory canal, leaving a cavity similar to that of mastoidectomy; iatrogenic cholesteatoma following middle ear surgery; complete opacity of the middle ear (either postoperatively or not). REFERENCES 1. Nemzek WR, Swartz JD. Temporal bone: inflammatory disease. In: Som PM, Curtin HD. Head and neck imaging. 4th ed. St Louis: Mosby; 2003. p. 1184–99. 2. Gebrim EMS. Ossos temporais. In: Gebrim EMS, Chammas MC, Gomes RLE. Radiologia e diagnóstico por imagem – cabeça e pescoço. 1ª ed. Rio de Janeiro: Guanabara Koogan; 2010. p. 52–60. 3. Olszewska E, Wagner M, Bernal-Sprekelsen M, et al. Etiopathogenesis of cholesteatoma. Eur Arch Otorhinolaryngol. 2004;261:6–24. 4. Fatterpekar GM, Doshi AH, Dugar M, et al. Role of 3D CT in the evaluation of the temporal bone. Radiographics. 2006;26 Suppl 1:S117–32. 5. Gaurano JL, Joharjy IA. Middle ear cholesteatoma: characteristic CT findings in 64 patients. Ann Saudi Med. 2004;24:442–7. 6. Razek AA, Huang BY. Lesions of the petrous apex: classification and findings at CT and MR imaging. Radiographics. 2012;32:151–73. 7. Prata AAS, Antunes ML, Abreu CEC, et al. Estudo comparativo entre achados radiológicos e cirúrgicos na otite média crônica. Arq Int Otorrinolaringol. 2011;15:72–8. 8. Moura MVT, Taranto DOL, Garcia MM. Colesteatoma: utilidade de sequência de difusão sem echo-planar. Radiol Bras. 2012;45:283–7. 9. De Foer B, Vercruysse JP, Bernaerts A, et al. Middle ear cholesteatoma: non-echo-planar diffusion-weighted MR imaging versus delayed gadolinium-enhanced T1-weighted MR imaging – value in detection. Radiology. 2010;255:866–72. 1. Trainees in Radiology and Imaging Diagnosis at Hospital Mater Dei, Belo Horizonte, MG, Brazil 2. MD, Radiologist, Coordinator for the Service of Radiology and Imaging Diagnosis of Hospital Mater Dei, Belo Horizonte, MG, Brazil 3. MDs, Radiologists, Hospital Mater Dei, Belo Horizonte, MG, Brazil Mailing Address: Dra. Ana Flávia Assis de Ávila Rua Miranda Ribeiro, 190, ap. 601, Vila Paris Belo Horizonte, MG, Brazil, 30380-660 E-mail: fauassis@hotmail.com Received September 15, 2012. Accepted after revision February 15, 2013. * Study developed at Hospital Mater Dei, Belo Horizonte, MG, Brazil. |
|
Av. Paulista, 37 - 7° andar - Conj. 71 - CEP 01311-902 - São Paulo - SP - Brazil - Phone: (11) 3372-4544 - Fax: (11) 3372-4554