INTRODUCTION
The term magnetic resonance cholangiopancreatography (MRCP) can be extended to a number of imaging acquisition techniques whose main characteristic is exploring a long T2 relaxation time of the fluids contained within the biliopancreatic tree for the formation of the image(1). As a general rule, MRCP studies are performed both under breath-hold (2D MRCP) and with respiratory triggering (3D MRCP). This strategy seeks to combine the inherent advantages from each of the two modalities, such as the short acquisition time of the 2D sequence and the higher spatial resolution obtained with the 3D techniques(2,3).
On account of its projectional nature, the signal from the gastrointestinal fluid can be superimposed over the biliopancreatic tree, limiting the diagnostic quality of the method(4). The signal from the gastrointestinal fluid may be abolished or reduced by three strategies as follows: prolonged fasting; intake of a negative oral contrast agent(5); or by means of reduction of gastric secretion with pharmaceuticals(6).
Negative contrast agents are paramagnetic solutions which promote reduction in signal intensity of the gastrointestinal fluid for shortening the T2 relaxation time at the magnetic resonance imaging (MRI). Among this group of substances one may find from industrialized products containing iron(7) to juices where the fruit mineral concentrations (for example: manganese in pineapple) is strong enough to reduce the T2 relaxation time(8,9). Paramagnetic oral contrast agents are expensive, not palatable and may cause side effects(7). As regards juices, differences in the mineral concentration required for suppressing the gastrointestinal liquid signal may occur in the household preparation of the solutions, as well as in the industrialized solutions, making them ineffective.
On the other hand, pharmaceuticals such as ranitidine are inexpensive, widely available, can be easily administered and with a low incidence of side effects(10), and do not require a medical prescription to be bought. At least one study has demonstrated ranitidine contribution in the evaluation of biliary tract with MRI, for reducing the gastroduodenal fluid signal and minimizing its interference on the visualization of the biliopancreatic tree(6). For that reason, the authors of the present study have decided to confirm the value of oral ranitidine in the evaluation of the biliopancreatic tree in MRCP studies.
MATERIALS AND METHODS
The present study was approved by the Committee for Ethics in Research of the institution. A term of free and informed consent was signed by all the individuals participating in the study.
A prospective, cross-sectional, observational and self-paired study was undertaken. The proposal for undergoing MRCP was opened to healthy and cooperative individuals aged above 18, who agreed in participating in the study. Any relative or absolute contraindication for MRI (for example: cardiac pacemaker, brain aneurysm clips, cochlear implants, claustrophobia), previous history of allergy to the drug and use of synergistic drugs (for example: diazepam, ketoconazole, clarithromycin, phenytoin and warfarin) were considered as exclusion criteria.
The MRCP scans were performed in 32 volunteers, 12 women and 20 men, with ages between 27 and 63 years, mean age of 32 years. No exclusion was observed.
Patients preparation
The following steps were undertaken:
First step - MRCP with no medication and after at least four-hour fasting, acquiring images with 2D and 3D techniques.
Second step - On the same day, MRCP after oral intake of negative contrast medium, acquiring images with 2D and 3D techniques. The negative contrast medium was a solution with 5 ml of gadolinium in 75 ml of water, as described in the literature(5).
Third step - MRCP under at least four-hour fasting and 12 hours after ingestion of 300 mg of ranitidine (peak of action) acquiring the images with 3D and 2D techniques. The mean time span between the first two steps and the third step was 30 days, ranging from 21 to 35 days.
Equipment and technique
All the scans were performed in a MRI equipment operating with a 1.5 T magnetic field, model Gyroscan Intera
® (Release 13) (Philips Medical Systems; Best, The Netherlands) equipped with a synergy sense body coil for signal emission and reception.
The parameters for MRCP images acquisition were identical in the three steps, namely:
- 3D MRCP: repetition time (TR) = 1433; echo time (TE) = 650; NEX = 1; matrix = 256 x 205; slice thickness = 1.6 mm; gap = 0.8 mm with 80% overlap; turbo factor (TF) = 144; flip angle of 90 degrees; field of view (FOV) = 512 x 512; echo spacing = 9.9 ms; bandwidth = 723 Hz; and acquisitions during the final expiratory phase utilizing respiratory triggering (RT). The mean acquisition time was five minutes.
- 2D MRCP: TR = 8000; TE = 800; NEX = 1; matrix = 228 x182; slice thickness = 4.0 cm; TF = 182; flip angle = 90 degrees; FOV = 380 x 380; bandwidth of 320 Hz; 6 radial coronal acquisitions centered on the bile duct, at an angle of 15 degrees and obtained with breath-hold. The time spent for each acquisition (slab of 4.0 cm) was 2 seconds, and for the entire sequence, less than 30 seconds.
Images evaluation
The images were independently and randomly evaluated by three different observers (observer 1, with 10-year experience in general radiology; observer 2, with 4-year experience in general radiology and 2-year experience in abdominal radiology; observer 3, with 4-year experience in general radiology). There was no consensual analysis in cases of interobserver disagreement.
The images were filed in DICOM format and were displayed on high-resolution displays utilizing the Osirix
®platform. In order to avoid learning bias, the identification of patients and fluid signal suppression technique were replaced by codes.
The 3D MRCP images were initially evaluated, also utilizing the millimetric sections of the source images and multiplanar reconstructions with the MIP technique. The observers were totally free to perform reconstructions in other planes, change the imaging windows according to their preferences as well as changing the reconstruction thickness with the MIP technique. Each evaluation session was held at an interval of at least two weeks.
The 2D acquisition images were later evaluated. Similarly to the previous evaluation sessions, the observers were free to change the images parameters as they found convenient.
Evaluation parameters
The following ductal segments were evaluated:
- Main right hepatic ducts branch: defined as the segment interposed between the ductal hilar confluence and its first-order branching.
- Main left hepatic ducts branch: defined as the segment interposed between the ductal hilar confluence and its first-order branching.
- Common hepatic duct: Segment interposed between the hilar confluence of the main left and right branches and the cystic duct insertion.
- Bile duct: defined as the ductal segment located below the cystic duct. The bile duct was arbitrarily defined as pancreatic (2 cm above its exit into the duodenum) and suprapancreatic (the remaining segment).
- Cystic duct: ductal structure extending from the gallbladder neck to the bile duct.
- Head of the pancreatic duct: up to 5 cm from the biliopancreatic junction(11).
- Body of the pancreatic duct: between 5 and 7 cm of the biliopancreatic junction(11).
- Tail of the pancreatic duct: 7 cm beyond the biliopancreatic junction(11).
- Gallbladder.
Qualitative images evaluation
The degree of gastrointestinal fluid signal suppression was evaluated according to the following scale:
1 - The hyperintense signal present in the stomach and in the duodenum impairs the analysis of the structure.
2 - The hyperintense signal present in the stomach and in the duodenum partially impairs the analysis of the structure.
3 - The hyperintense signal present in the stomach and in the duodenum does not impair the analysis of the structure.
4 - There is no signal present inside the stomach and the duodenum.
The ductal visualization was evaluated according to the following scale:
0 - The duct is not visualized.
1 - The duct is partially visualized and defined.
2 - The duct is clearly visualized and defined.
Statistical analysis
The sum of the scores assigned by the three observers for the
visualization variable (ranging between 0 and 2), so as the score value was a minimum of 0 and a maximum of 6. The same was done to evaluate the
degree of gastrointestinal fluid signal suppression (ranging between 1 and 4), where the sum of scores could range from 3 to 12. Such a method was applied for both image acquisition techniques (2D and 3D) and for the three gastroduodenal fluid signal suppression techniques (fasting, ranitidine and oral contrast).
The two-way analysis of variance (ANOVA) was utilized to verify significant differences between acquisition techniques (2D and 3D). In the case of statistical significance, the Tukey multiple comparison test was utilized.
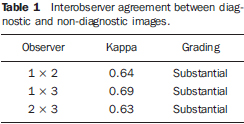
The interobserver agreement was evaluated by means of the kappa (κ) test. Value of κ lower than 0.4 indicate low agreement; values between 0.41 and 0.60 indicate moderate agreement; between 0.61 and 0.80 indicate substantial agreement; above 0.81 indicate excellent agreement(12).
The established significance level was
p > 0.05.
RESULTS
Interobserver agreement
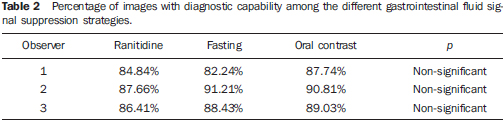
The interobserver agreement in relation to the degree of gastrointestinal fluid signal suppression was considered as being substantial, ranging between κ = 0.63 and κ = 0,73. For the qualitative assessment of the visualization of biliopancreatic duct segments, the interobserver agreement was moderate, ranging between κ = 0.41 and κ = 0.48. The images were considered as having diagnostic effectiveness when the biliopancreatic tree was partially or completely visualized (visualization score 1 and 2). On the other hand, images without diagnostic effectiveness were those where a determined biliopancreatic duct segment was not identified (score 0). As the images were grouped as "with diagnostic effectiveness" (visualization scores 1 and 2) and "without diagnostic effectiveness" (visualization category 0), the agreement about visualization of the biliopancreatic tree became substantial (Table 1). No statistically significant difference was observed in relation to the number of images with diagnostic effectiveness among the three techniques (fasting, ranitidine and oral negative contrast) (Table 2).
The utilization of oral contrast agent was the strategy by which the highest suppression scores were achieved (Figures 1 and 2), and the difference with other gastrointestinal fluid signal suppression strategies (fasting and ranitidine) were statistically significant for any of the two acquisition techniques (2D x 3D) (
p = 0.02). No statistically significant difference was observed between the scores obtained with ranitidine and fasting in both image acquisition techniques. The effect of the negative contrast on all segments is presented on Figure 3, where the scores for the three strategies for gastrointestinal fluid signal suppression with both acquisition techniques are represented.
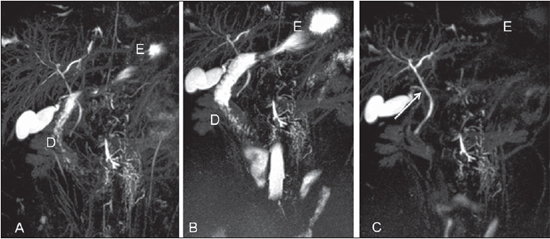
Figure 1. 3D MIP reconstructions with ranitidine (A), fasting (B) and with gadolinium solution (C). Decreased signal of the stomach (E) and of the duodenum (D) with the utilization of negative oral contrast agent. The cystic duct (arrow on C) was better characterized with oral gadolinium solution.

Figure 2. Comparison between strategies for gastrointestinal fluid signal reduction at 3D MIP reconstructions with ranitidine (A), fasting (B) and with gadolinium solution (C). The gastrointestinal fluid signal is similar with the ranitidine and fasting strategies. Arrow on C identifies the cystic duct. E, stomach; D, duodenum.
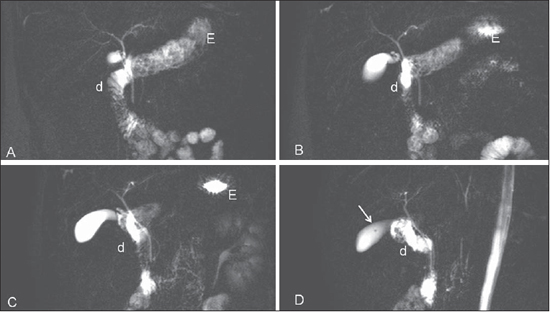
Figure 3. 2D sequence after fasting where, in spite of angled acquisitions, the cystic duct superposition persisted. Detail of small vesicular polyp on image D (arrow). E, stomach; d, duodenum.
Ten segments of the biliopancreatic tree were evaluated. All the 32 patients were evaluated six times (three times on 3D acquisitions and three times on 2D acquisitions). Thus, each observer performed 1,920 image analyses. The scores 1 and 2 (when the gastrointestinal fluid signal partially or totally impairs the biliopancreatic tree evaluation) occurred in only 3.2% of all evaluations, being most commonly observed in the analysis of the cystic duct on 2D acquisitions by the less experienced observers (Figure 4).
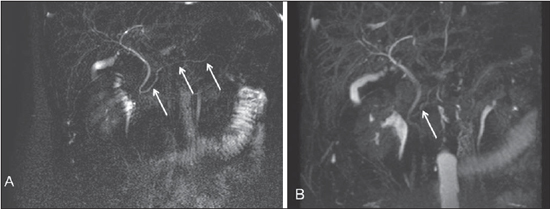
Figure 4. All segments of the pancreatic duct are identified at the 2D acquisition with gadolinium solution (arrows on A). At the 3D MIP sequence (B) only its cephalic segment is partially identified (arrow).
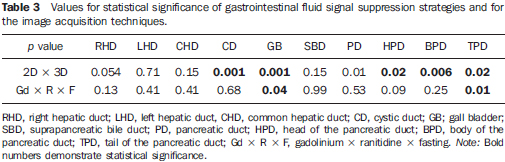
The quality of ductal visualization kept a closer relationship with the acquisition technique (2D x 3D) than with the gastrointestinal fluid signal suppression strategy (Table 3).
For the right, left and common hepatic ducts, and the suprapancreatic segment of the bile duct, there was neither statistically significant difference between the acquisition techniques (2D x 3D) nor between the gastrointestinal fluid signal suppression strategies.
For the cystic duct and the gallbladder, the acquisitions with the 3D technique achieved higher visualization scores.
For the pancreatic segment of the bile duct, and for the entire extent of the pancreatic duct, the 2D acquisition surpassed the 3D acquisition technique, and such difference was statistically significant (Figure 5).
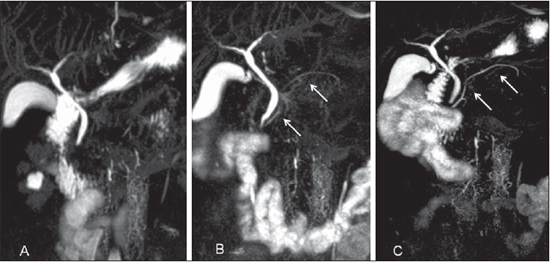
Figure 5. 3D MIP reconstructions with the utilization of ranitidine (A); oral gadolinium solution (B); and after fasting (C). Observe the difference in the visualization degree of the pancreatic duct in the same patient (arrows on B and C).
The evaluation of the gallbladder and cystic duct was significantly superior with ingestion of the gadolinium solution. In the remaining segments of the biliopancreatic tree the ductal visualization was similar among the three gastrointestinal fluid signal suppression techniques (Table 3).
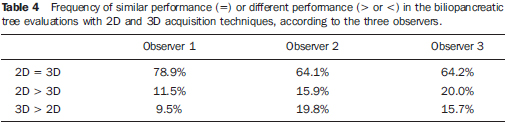
For the three observers, in the majority of images, the 2D and 3D acquisition techniques presented similar performances in the visualization of the biliopancreatic tree (Table 4).
In all studied ductal segments, the gastrointestinal fluid signal suppression strategy with ingestion of ranitidine did not result in a better evaluation of the biliopancreatic tree as compared with the other two strategies, and there was no statistically significant difference in comparison with the scans performed after fasting only. In the evaluation of the tail of the pancreatic duct, a worse definition was observed on the images obtained after the utilization of ranitidine (Figure 6), as compared with the images obtained with other strategies for gastrointestinal fluid signal suppression (Table 3).
DISCUSSION
MRCP is the main non-invasive imaging method utilized in the evaluation of biliopancreatic disorders. Its accuracy is similar to that obtained at endoscopic retrograde cholangiopancreatography in the diagnosis of choledocholithiasis, biliary obstruction and sclerosing cholangitis(13,14), without the potential risks involved in such invasive procedure(15). In order to obtain such expected results, it is essential to assure a good imaging quality, which is expressed by the degree of ductal visualization(16). The inappropriate characterization of the biliopancreatic tree ducts may originate from the presence of motion artifacts caused by peristaltic and respiratory movements and enteric fluid overlap(17). In the present study, the authors have tried to demonstrate the results achieved in terms of ductal visualization at MRCP when different strategies are adopted, with the purpose of minimizing the superposition of gastrointestinal fluid over the biliary and pancreatic trees.
Among such strategies, the adoption of gadolinium solution in water was the best form to abolish the signal from the gastrointestinal fluid. As regards the pharmacological strategy utilizing oral ranitidine, the results of the present study were not similar to those reported by a previous study(5). No significant difference was observed as regards reduction in signal between scans performed after fasting and after ingestion of 300 mg ranitidine.
Ranitidine reduces the gastric acid secretion thanks to its action on the H2 receptors, reducing the hydrogen protons concentration in the gastric juice(10). On their turn, gastric juices are utilized in the formation of the images at the MRI. There are studies demonstrating a linear relationship between the signal intensity measured by MRI and local concentration of hydrogen(18,19). Probably, there was a difference in the concentration of such ions in the gastric juice as such difference was not expressed in terms of amount of signal of the gastrointestinal fluid perceived by the observers. Another point to be considered is the effect of ranitidine on the gastric motility. According some authors, there is a prokinetic effect, accelerating the gastric emptying, while for others there is an opposed effect(20-23), which can influence the results regarding the amount of fluid inside the gastric cavity. Such point is still controversial and should be further investigated in new studies about gastroduodenal motility. The ranitidine dose and its intake timing represent other issues which might have influenced its effectiveness in obtaining a more expressive reduction of gastric secretion. However such variables were compatible with those utilized in other studies and supported by their pharmacological action(6,10).
A worsening in the visualization of the caudal segment of the pancreatic duct was observed with the utilization of ranitidine in both acquisition techniques (2D and 3D). Bowers et al. have also mentioned a non-significant reduction in the visualization of the pancreatic duct in their study, but those authors did not make any further comment on such a finding(6). The present study authors' hypothesis is that the basal gastric acid secretion maintains some stimulus over the exogenous pancreatic bicarbonate secretion, which would cause fluid to be present in the pancreatic ductal lumen, and consequential MRI signal on cholangiographic sequences. On the contrary, the gastric secretion suppression caused by ranitidine would limit such an effect.
Fluid superposition impairing the ductal visualization was not a frequent event, identified in only 3.2% of the scans. Such finding was more common for the cystic duct in 2D acquisitions. These scores were most frequently observed in the analyses undertaken by the less experienced observers. As far as one could observe, the present study is the first to identify the frequency in which the fluid superposition limits the ductal visualization, and also the first to relate it to the observers' experience degree.
The visualization score was rather related to the acquisition modality and location of the evaluated segment. According to the present study results, the sequences obtained with the 3D and RT techniques improved the degree of visualization of the cystic duct and of the gallbladder. In the remaining segments, the visualization scores obtained with breath-hold sequences were equivalent or higher than those obtained with 3D sequences. Such aspect was mostly observed on the pancreatic duct images. According to some authors, there is superiority in the visualization of this segment as its anatomic plane is contained within the acquisition block, like in the 2D technique(24). Furthermore, reconstructions with MIP technique from images obtained with the 3D technique may exacerbate motion artifacts(25). Considering such aspects and the shorter acquisition time for imaging with the 2D breath-hold technique, it is justifiable to initiate the MRCP scan with this acquisition technique. In those cases where some ductal segment, crucial for diagnosis definition, is not appropriately represented with the 2D technique, the scan may be supplemented with images acquired with RT and the 3D technique. In cases where fasting alone was the strategy utilized for gastrointestinal fluid signal suppression, the use of negative oral contrast agent would have been necessary in three (9%) of the 32 cases for observer 1, in none for observer 2, and in five cases (15%) for observer 3. Gastrointestinal fluid signal suppression would be useful particularly in the evaluation of the cystic duct only in 2D acquisitions.
In summary, the three observers substantially agreed in relation to the fact that the signal from the enteric juice is rarely a factor which affects the biliopancreatic tree visualization degree as the scan is performed after fasting. For that reason, the authors consider, similarly to other authors, that the utilization of negative contrast agent may be dispensable in MRCP scans(2). However, there is no consensus in the literature with respect to this theme(9). Some authors have put forward opinions which are based on their experience, rather than on hard evidences, indicating that the use oral contrast agent is not necessary(1). On the other hand, others have demonstrated the usefulness of such a strategy to improve the visualization of the biliopancreatic tree(5,17). It is important to notice that the latter have not utilized the radial sequence in the 2D breath-hold acquisitions, or have not performed the 3D sequences with RT in the routine evaluation of the biliopancreatic tree in their studies, contrarily to what was done in the present study, and which reduces the undesired superposition of the gastric juice with the biliopancreatic ducts. That would explain the discrepancy obtained in the present study as compared with previous ones, towards considering the use of oral contrast unnecessary, since the different angles of radial and volumetric acquisitions avoid the fluid superposition on the biliopancreatic tree mapping.
The present study faced some limitations. The first one is the fact that patient fasting time was not controlled; only a minimum fasting time of at least four hours between the last meal and the scan was established; some patients may have fasted for longer than four hours, as it occurs in the daily practice. The second limitation was the fact that volunteers' diet was not controlled. Some types of food can retard the gastric emptying, which may eventually reduce the efficacy of fasting as a strategy for reducing ductal superposition; however, limiting the intake of certain foods is not common in the patient preparation instructions for MRCP scans. The third limitation was the fact that no antiperistaltic drugs were utilized. Sodickson et al. report the finding of motion artifacts caused by peristaltic movements under the form of noise dispersed throughout the image on 3D MRCP acquisitions, leading to poor definition of ductal contours(26). This may have compromised image quality in 3D acquisitions; on the other hand, considering that some patients are sensitive to scopolamine, avoiding the utilization of such type of drug increases the tolerance to the procedure. Finally, the new respiratory-triggered techniques by means of diaphragmatic motion monitoring (PACE)(27) seem to be superior to that utilized for the present study. This may have favored the 2D sequences in the present study, i.e., adopting more updated RT techniques might have improved the quality of the images obtained in 3D and make them better than those obtained with the breath-hold technique. On the other hand, it should be considered that new techniques for breath-hold acquisition might equally optimize results in the biliopancreatic tree evaluation by MRCP.
In the evaluation of conditions affecting the pancreatic and biliary tract, MRCP has been playing an increasing role on account of its many advantages and complementarity with ultrasonography(28-30). Establishing a MRCP exam protocol that makes it simpler, faster and more efficient without impairing its diagnostic capability is important, and the present study is aimed at contributing in such sense.
Finally, the present study demonstrates that the utilization of oral ranitidine does not bring significant benefits to the image quality in MRCP studies. It is possible to perform the scan in a simplified way and with good quality by utilizing the breath-hold acquisition technique and after fasting. By dispensing with the use of time consuming acquisition sequences with RT and gastrointestinal fluid signal reduction strategies (either pharmacological or involving the use of oral contrast agents), and utilizing fast breath-hold sequences, one should save time and resources.
REFERENCES
1. Bearcroft PW, Lomas DJ. Magnetic resonance cholangiopancreatography. Gut. 1997;41:135-7.
2. Larena JA, Astigarraga E, Saralegui I, et al. Magnetic resonance cholangiopancreatography in the evaluation of pancreatic duct pathology. Br J Radiol. 1998;71:1100-4.
3. Lomas DJ, Bearcroft PW, Gimson AE. MR cholangiopancreatography: prospective comparison of a breath-hold 2D projection technique with diagnostic ERCP. Eur Radiol. 1999;9:1411-7.
4. Irie H, Honda H, Kuroiwa T, et al. Pitfalls in MR cholangiopancreatographic interpretation. Radiographics. 2001;21:23-37.
5. Chan JH, Tsui EY, Yuen MK, et al. Gadopentetate dimeglumine as an oral negative gastrointestinal contrast agent for MRCP. Abdom Imaging. 2000;25:405-8.
6. Bowes MT, Martin DF, Melling A, et al. Single dose oral ranitidine improves MRCP image quality: a double-blind study. Clin Radiol. 2007;62:53-7.
7. Galvão Filho MM, D'Ippolito G, Borri ML, et al. Uso do contraste oral negativo em exames de colangiografia por ressonância magnética. Radiol Bras. 2002;35:267-71.
8. Riordan RD, Khonsari M, Jeffries J, et al. Pineapple juice as a negative oral contrast agent in magnetic resonance cholangiopancreatography: a preliminary evaluation. Br J Radiol. 2004;77:991-9.
9. Sanchez TA, Elias J Jr, Colnago LA, et al. Clinical feasibility of açaí (Euterpe oleracea) pulp as an oral contrast agent for magnetic resonance cholangiopancreatography. J Comput Assist Tomogr. 2009;33:666-71.
10. Santos DRDS, Silva LR. Farmacologia clínica das drogas antiulcerosas e antidispépticas. In: Silva P, organizador. Farmacologia. Rio de Janeiro, RJ; 2010. p. 881-7.
11. Chu ZQ, Ji Q, Zhang JL. Orally administered lemon/orange juice improved MRCP imaging of pancreatic ducts. Abdom Imaging. 2010;35:367-71.
12. Bland JM, Altman DG. Measuring agreement in method comparison studies. Stat Methods Med Res. 1999;8:135-60.
13. Romagnuolo J, Bardou M, Rahme E, et al. Magnetic resonance cholangiopancreatography: a meta-analysis of test performance in suspected biliary disease. Ann Intern Med. 2003;139:547-57.
14. Vitellas KM, Enns RA, Keogan MT, et al. Comparison of MR cholangiopancreatographic techniques with contrast-enhanced cholangiography in the evaluation of sclerosing cholangitis. AJR Am J Roentgenol. 2002;178:327-34.
15. Scheiman JM, Carlos RC, Barnett JL, et al. Can endoscopic ultrasound or magnetic resonance cholangiopancreatography replace ERCP in patients with suspected biliary disease? A prospective trial and cost analysis. Am J Gastroenterol. 2001;96:2900-4.
16. Hundt W, Petsch R, Scheidler J, et al. Clinical evaluation of further-developed MRCP sequences in comparison with standard MRCP sequences. Eur Radiol. 2002;12:1768-77.
17. Coppens E, Metens T, Winant C, et al. Pineapple juice labeled with gadolinium: a convenient oral contrast for magnetic resonance cholangiopancreatography. Eur Radiol. 2005;15:2122-9.
18. Heverhagen JT, Müller D, Battmann A, et al. MR hydrometry to assess exocrine function of the pancreas: initial results of noninvasive quantification of secretion. Radiology. 2001;218:61-7.
19. Punwani S, Gillams AR, Lees WR. Non-invasive quantification of pancreatic exocrine function using secretin-stimulated MRCP. Eur Radiol. 2003;13:273-6.
20. Kemmostsu O, Mizushima M, Morimoto Y, et al. Effect of preanesthetic intramuscular ranitidine on gastric acidity and volume in children. J Clin Anesth. 1991;3:451-5.
21. Cucchiara S, Raia V, Minella R, et al. Ultrasound measurement of gastric emptying time in patients with cystic fibrosis and effect of ranitidine on delayed gastric emptying. J Pediatr. 1996;128:485-8.
22. Madsen JL, Graff J. Effects of the H2-receptor antagonist ranitidine on gastric motor function after a liquid meal in healthy humans. Scand J Clin Lab Invest. 2008;68:681-4.
23. Parkman HP, Urbain JL, Knight LC, et al. Effect of gastric acid suppressants on human gastric motility. Gut. 1998;42:243-50.
24. Chen RC, Lin KY, Lii JM, et al. MR cholangiopancreatography: prospective comparison of 3-dimensional turbo spin echo and single-shot turbo spin echo with ERCP. J Formos Med Assoc. 2003;102:172-7.
25. Morrin MM, Farrell RJ, McEntee G, et al. MR cholangiopancreatography of pancreaticobiliary diseases: comparison of single-shot RARE and multislice HASTE sequences. Clin Radiol. 2000;55:866-73.
26. Sodickson A, Mortele KJ, Barish MA, et al. Three-dimensional fast-recovery fast spin-echo MRCP: comparison with two-dimensional singleshot fast spin-echo techniques. Radiology. 2006;238:549-59.
27. Morita S, Ueno E, Suzuki K, et al. Navigator-triggered prospective acquisition correction (PACE) technique vs. conventional respiratory-triggered technique for free-breathing 3D MRCP: an initial prospective comparative study using healthy volunteers. J Magn Reson Imaging. 2008;28:673-7.
28. Arruda ECM, Coelho JCU, Yokochi JM, et al. O papel da colangiografia por ressonância magnética na avaliação da anatomia biliar em doadores de transplante hepático intervivos. Radiol Bras. 2008;41:361-5.
29. Sales DM, Santos JEM, Shigueoka DC, et al. Correlação interobservador das alterações morfológicas das vias biliares em pacientes com esquistossomose mansoni pela colangiorressonância magnética. Radiol Bras. 2009;42:277-82.
30. Souza LRMF, Rodríguez FB, Tostes LV, et al. Avaliação por imagem das lesões císticas congênitas das vias biliares. Radiol Bras. 2012;45:113-7.
1. MDs, Radiologists, Fellows Master degree, Abdominal Imaging, Department of Diagnostic Imaging, Escola Paulista de Medicina - Universidade Federal de São Paulo (EPM-Unifesp), São Paulo, SP, Brazil.
2. Fellows, MDs, Radiologists, Hospital São Luiz, São Paulo, SP, Brazil.
3. Associate Professor, Department of Diagnostic Imaging, Escola Paulista de Medicina - Universidade Federal de São Paulo (EPM-Unifesp), São Paulo, SP, Brazil.
Mailing Address:
Dr. Giuseppe D'Ippolito
Departamento de Diagnóstico por Imagem - EPM-Unifesp
Rua Napoleão de Barros, 800, Vila Clementino
São Paulo, SP, Brazil, 04024-002
E-mail: giuseppe_dr@uol.com.br
Received October 8, 2012.
Accepted after revision January 4, 2013.
* Study developed at Department of Diagnostic Imaging, Escola Paulista de Medicina - Universidade Federal de São Paulo (EPM-Unifesp), São Paulo, SP, Brazil.
 Vol. 46 nº 2 - Mar. / Apr. of 2013
Vol. 46 nº 2 - Mar. / Apr. of 2013