INTRODUCTION
Lethal midline granuloma syndrome (LMG) comprises a condition whose diagnosis is difficult to be made because of the wide array of related diseases and nonspecific symptoms. Midline destructive lesions of the face were first described in 1897, and later a variety of terms were coined to describe them. A factor that is common to all of such lesions is the development of an ulcerative/vegetative process culminating with destruction of the nasal region, resulting in functional and cosmetic deformity(1).
The majority of cases involve natural killer/T cell non-Hodgkin's lymphoma and Wegener's granulomatosis, and epidermoid carcinoma should be considered as differential diagnosis since it is the most prevalent malignant neoplasm of the head, corresponding to 5% of all tumors occurring in the world population(2,3).
CASE REPORT
A female, black, 58-year-old patient presented a small ulcerative lesion in the glabellar region in February/2012, progressing with fast growth and a predominantly vegetative behavior.
Three months after the first evaluation, the patient was admitted for investigation of an already large-sized lesion (Figure 1). Laboratory tests did not demonstrate any relevant findings, and fine-needle aspiration biopsy was inconclusive. Skull computed tomography (CT) (Figure 2) demonstrated the presence of a vegetative, heterogeneous lesion in association with extensive bone destruction predominantly affecting the nasal septum and the frontal sinus.
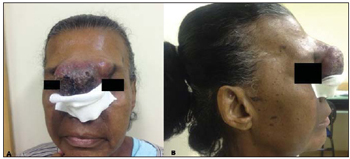
Figure 1. A: Large-sized lesions with epicenter in the glabella, showing a relevant vegetative component associated with ulcerations with necrotic appearance. B: Lateral view.
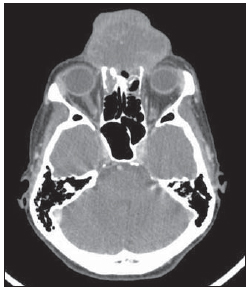
Figure 2. Contrast-enhanced skull computed tomography. Axial section demonstrate heterogeneous vegetative lesion with soft tissue density, extensive bone destruction and subtle contrast-enhancement, particularly in the periphery of the lesion.
Complementary magnetic resonance imaging (MRI) (Figure 3) performed 13 days after the CT scan demonstrated the presence of heterogeneous isointensity at T1-weighted, and mild hyperintensity at T2-weighted and FLAIR sequences, with subtle gadolinium enhancement. A synchronous lesion similar to the previously mentioned one was also observed, but without contiguity and with close contact with the maxillary palatine process.
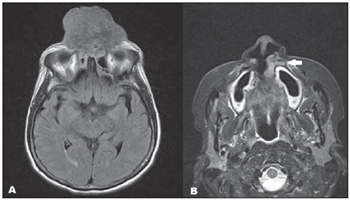
Figure 3. A: Axial, FLAIR sequence demonstrating the presence of heterogeneous, mildly hyperintense lesion with significant midline destruction.B: Axial, T2-weighted STIR image demonstrating a small synchronous lesion (arrow) with subtle hypersignal intensity, adjacent to the lateral wall of the left nasal ostium, in close contact with the maxillary palatine process. Biopsy revealed the presence of epidermoid carcinoma.
At follow-up, biopsy was performed (Figure 4), including the synchronous lesions, with anatomopathological results compatible with epidermoid carcinoma.
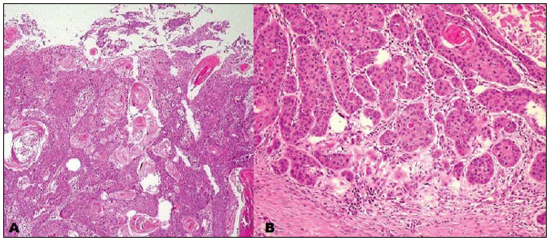
Figure 4. A: Infiltration of the dermis and epidermis by tumor cells with intermingled keratin pearls characteristic of epidermoid carcinoma. B: Area of lower differentiation of the tumor with a lower number of keratin pearls.
LMG is rarely found and hardly diagnosed because of nonspecific symptoms, many times requiring several biopsies for a correct diagnosis(4).
Main diseases implied in this clinical condition include natural killer/T-cell non-Hodgkin's lymphoma and Wegener's granulomatosis, but with a wide range of differential diagnoses, particularly epidermoid carcinoma, like in the present case.
Epidermoid carcinoma originates from suprabasal keratinocytes, predominantly affecting men, with incidence peak in the age range between 50 and 70 years. Risk factors are related to the disease location, thus smoking and alcohol consumption represent main risk factors for mucosal lesions, while ultraviolet radiation exposure, chronic ulcers and fistulas, for skin lesions. Amongst head and neck neoplasms, epidermoid carcinoma is the most common malignant neoplasia, corresponding to 5% of cancer cases.
The symptoms are generally insidious and are related to the lesion's site of origin. Metastases usually involve lymph nodes, and the treatment of choice is surgery in association with radiotherapy and chemotherapy in selected cases.
Both at CT and MRI, epidermoid carcinomas are not distinctive from other lesions, typically appearing with irregular margins, bone destruction and heterogeneous contrast enhancement. In a study developed by Groell et al.(5) with 27 patients with epidermoid carcinoma, it was suggested that delayed CT images acquired at 180 seconds following contrast injection would allow a better delimitation of the lesion.
It is interesting to note that 8%(2) of epidermoid carcinomas present as synchronous lesions, like in the present case, constituting an indication for positron emission tomography. Recent studies highlight the utilization of diffusion weighted MRI in the evaluation of LMG, demonstrating that apparent diffusion coefficient values < 1.22 x 10
-3 mm
2/s(6,7) are suggestive of the presence of malignant lesions, and values < 0.84 x 10
-3 mm
2/s(6,7) are more compatible with lymphomas. In the present case, with the same protocol utilized in those studies, the lesion presented an apparent diffusion coefficient of 0.91 x 10
-3 mm
2/s, corroborating the previously described findings.
Differential diagnoses include principally, among others, natural-killer/T-cell non-Hodgkin's lymphoma, with highest prevalence in Asian individuals aged above 50 and in association with Epstein-Barr virus. The differentiation of the disease by means of imaging methods remains difficult. Lymph node enlargement represents an uncommon finding, bone involvement is less severe than in cases of epidermoid carcinoma, and involvement of the anterior wall of the maxillary sinus is rarely observed(8).
Wegener's granulomatosis is another possible diagnosis to be considered, preferentially affecting men aged between 40 and 50 years, and classically presenting with airways lesion, glomerulonephritis and disseminated vasculitis. In such cases, c-ANCA testing plays a relevant role in the diagnosis.
Other differential diagnoses include polymorphic reticulosis, tuberculosis, leishmaniasis, cocaine abuse, giant cell granuloma, cholesterol granuloma and lobular capillary hemangioma, these last three conditions being associated with trauma.
Finally, the diagnosis of epidermoid carcinoma should be considered in LMG, since it is the main head and neck neoplasm. Additionally, the presence of synchronous involvement should be investigated.
REFERENCES
1. Parker NP, Pearlman AN, Conley DB, et al. The dilemma of midline destructive lesions: a case series and diagnostic review. Am J Otolaryngol. 2010;31:104-9.
2. Dammann F, Horger M, Mueller-Berg M, et al. Rational diagnosis of squamous cell carcinoma of the head and neck region: comparative evaluation of CT, MRI, and 18FDG PET. AJR Am J Roentgenol. 2005;184:1326-31.
3. Mendonça VF, Carvalho ACP, Freitas E, et al. Tumores malignos da cavidade nasal: avaliação por tomografia computadorizada. Radiol Bras. 2005;38:175-80.
4. Lessa MM, Goto EY, Voegels RL, et al. Granuloma de linha media: revisão de 17 casos. Arq Int Otorrinolaringol. 2001;5(1). [acessado em 29 de maio de 2012]. Disponível em: http://www.arquivosdeorl. org.br/conteudo/acervo_port.asp?id=146
5. Groell R, Doerfler O, Schaffler GJ, et al. Contrast-enhanced helical CT of the head and neck: improved conspicuity of squamous cell carcinoma on delayed scans. AJR Am J Roentgenol. 2001;176:1571-5.
6. Wang J, Takashima S, Takayama F, et al. Head and neck lesions: characterization with diffusion-weighted echo-planar MR imaging. Radiology. 2001;220:621-30.
7. Gonçalves FG, Ovalle JP, Grieb DFJ, et al. Diffusion in the head and neck: an assessment beyond the anatomy. Radiol Bras. 2011;44:308-14.
8. Ooi GC, Chim CS, Liang R, et al. Nasal T-cell/natural killer cell lymphoma: CT and MR imaging features of a new clinicopathologic entity. AJR Am J Roentgenol. 2000;174:1141-5.
1. MD, Resident of Radiology, Hospital Universitário Clementino Fraga Filho - Universidade Federal do Rio de Janeiro (HU-CFF-UFRJ), Rio de Janeiro, RJ, Brazil.
2. Assistant Professor, Department of Radiology, Universidade Federal do Rio de Janeiro (UFRJ), Head of the Unit of Radiology and Imaging Diagnosis, Hospital Universitário Clementino Fraga Filho - Universidade Federal do Rio de Janeiro (HUCFF-UFRJ), Rio de Janeiro, RJ, Brazil.
3. MD, Resident of Medical Practice, Hospital Federal da Lagoa, Rio de Janeiro, RJ, Brazil.
4. MD, Radiologist, Head of the Unit of Computed Tomography, Hospital Federal da Lagoa, Rio de Janeiro, RJ, Brazil.
Mailing Address:
Dr. Bruno Niemeyer de Freitas Ribeiro
Estrada do Capenha, 1431, ap. 202, bloco 01, Freguesia, Jacarepaguá
Rio de Janeiro, RJ, Brazil, 22743-041
E-mail: bruno.niemeyer@hotmail.com
Received August 4, 2012.
Accepted after revision September 11, 2012.
* Study developed at Hospital Universitário Clementino Fraga Filho - Universidade Federal do Rio de Janeiro (HUCFF-UFRJ), Rio de Janeiro, RJ, Brazil.
 Vol. 45 nº 6 - Nov. / Dec. of 2012
Vol. 45 nº 6 - Nov. / Dec. of 2012



