Radiologia Brasileira - Publicação Científica Oficial do Colégio Brasileiro de Radiologia
AMB - Associação Médica Brasileira CNA - Comissão Nacional de Acreditação
 Vol. 45 nº 6 - Nov. / Dec. of 2012
Vol. 45 nº 6 - Nov. / Dec. of 2012
|
REVIEW ARTICLES
|
|
Novel functional methods in the evaluation of breast lesions |
|
|
Autho(rs): Filipe Ramos Barra1; Renato Ramos Barra2; Alaor Barra Sobrinho3 |
|
|
Keywords: Mammography; Breast scintigraphy; Breast neoplasms; Magnetic resonance imaging. |
|
|
Abstract: INTRODUCTION
Breast cancer is the most prevalent type of cancer in women worldwide, and its incidence has increased over the last years. Except for non-melanoma skin tumors, breast cancer is the most frequent type of cancer in women among the population of the Southeastern, Center-Western and Northeastern regions Brazil(1). In spite of being considered as a cancer with good prognosis, provided it is correctly diagnosed and treated, the country-wide breast cancer mortality rates have decreased but remain high, probably because the diagnosis is made only at late stages of the disease. In developed countries, the average five-year survival rate is about 85%, while in developing countries it is around 60%(1). One of the factors contributing to a longer survival is the correct staging and early detection of metastases, which can be achieved with PET/CT, bone scintigraphy or multidetector computed tomography(2,3). Mammography is the only morphological imaging method with a proved impact on the reduction of breast cancer mortality(4). However, as a two-dimensional method, mammography is less sensitive to differentiate tissues, particularly in young women and in those with dense breasts(5,6). Additionally, it does not present a good performance in surgical planning, with a rate of postoperative residual lesion ranging between 30% and 60%(7). The breasts density is directly related to the utilization of hormone replacement therapy, and indirectly related to age, parity and body mass index(8). In women with dense breasts, there is an increase in the number of dubious (probably benign) findings, and in the rate of false-positive results determining unnecessary biopsies(9). Even with the utilization of computer-aided detection (CAD) systems, the mammography performance is not perfect, particularly for detecting architectural distortion and nodules(10). Ultrasonography plays a relevant role as a tool complementary to mammography and clinical examination. Besides detecting mammographically occult lesions, ultrasonography is useful in the differentiation between cystic and solid nodules and in the characterization of the degree of suspicion of solid nodules. The utilization of morphometric parameters provides a better differentiation between benign and malignant lesions. Amongst such parameters, the depth-to-width ratio should be highlighted for its good performance and facility of application(11). Magnetic resonance imaging (MRI) has shown excellent results in the detection and characterization of breast lesions. Such method has brought along new concepts related to angiogenesis and vascular supply. In the daily clinical practice, the main indications for MRI include evaluation of inconclusive sonographic or mammographic findings, surgical planning and evaluation of treatment response(12). Diffusion-weighted sequences allow the differentiation between some benign and malignant lesions(13). So one questions whether only the morphological analysis provided by mammography is sufficient or whether an evaluation of the vascular and metabolic behavior of the lesions is required(14,15). Currently, MRI is the best method to assess dense breasts, women at high (personal or familial) risk, dubious mammographic findings, pre-neoadjuvant chemotherapy staging, and treatment response(15-17). Limitations of MRI include high cost, long interpretation time, contraindications, besides the not always easy correlation of the method with mammography and ultrasonography because of different positionings. Novel functional evaluation technologies have been developed to supply the needs in this field. The authors have reviewed studies published about contrast-enhanced digital mammography and molecular imaging, describing the techniques, commenting their main features, advantages and disadvantages, besides briefly reporting their initial experience. CONTRAST-ENHANCED DIGITAL MAMMOGRAPHY Technological developments of digital detectors and X-ray tubes, as well as studies about the dual-energy technique have allowed the development of contrast-enhanced digital mammography, also called contrast-enhanced spectral mammography or simply contrast-enhanced mammography(18). Two contrast-enhanced mammography techniques are described, namely the dual-energy technique and the temporal subtraction technique(18,19). Independently from the technique, iodinated contrast agent is utilized at the same dose utilized for computed tomography (1-2 ml/kg), preferably injected by an injection pump at a flowing rate of 3 to 5 ml/s, with the same contraindications and risks described for that method, and requiring similar preparation(20,21). Contrast-enhanced mammography with temporal subtraction is similar to angiography. Before contrast injection, a mask image is acquired for subtraction of the subsequent contrast-enhanced images. The contrast agent is administered with the breast compressed. The whole procedure lasts about five minutes and may be susceptible to motion artifacts. Similarly to MRI, such technique allows the analysis of the kinetic curve of breast lesions enhancement, but only one breast can be evaluated at a single view(22). The dual-energy technique explores the different X-ray attenuations produced by the different materials. The digital mammography system is adapted with insertion of a copper filter, so it is possible to obtain an X-ray spectrum with energy above the K edge of the iodine (33.2 keV) for high-energy images, generally between 45 and 49 kV. The iodinated contrast agent is injected with the patient seated and breasts without compression. A pair of low- and high-energy images is acquired for each view, with a mean duration of 10 seconds per view. The images are acquired over a period of 2 to 7 minutes, with basis on the previous MRI findings. The high-energy image radiation dose corresponds to about 20% of the dose obtained at conventional mammography, thus there is a total increment of only 20% over the final dose of the procedure(20). The technique advantage is represented by the possibility of acquiring images on different planes, besides compressions and magnifications from both breasts with a single contrast injection. However, the analysis of the kinetic curve of enhancement is not feasible with this technique. As compared with digital mammography, contrast-enhanced mammography presents a higher sensitivity (93% versus 78%), with good specificity (83%)(20). As the images were analyzed by multiple observers, an increase of 20% in sensitivity could be observed, particularly in the cases of dense breasts. Some tumors only could be identified on the contrast-enhanced images(23). In the authors' experience with the GE SenoBright system (GE Healhtcare; Buc, France) a considerable enhancement of suspicious lesions (ductal carcinoma in situ, invasive carcinoma, and lobular carcinoma) was observed, with a good correlation with ultrasonography and principally with MRI. In women with dense breasts, high-energy images highlight suspicious lesions, as demonstrated on Figures 1 and 2, allowing their retrospective identification at non-contrast-enhanced mammography. 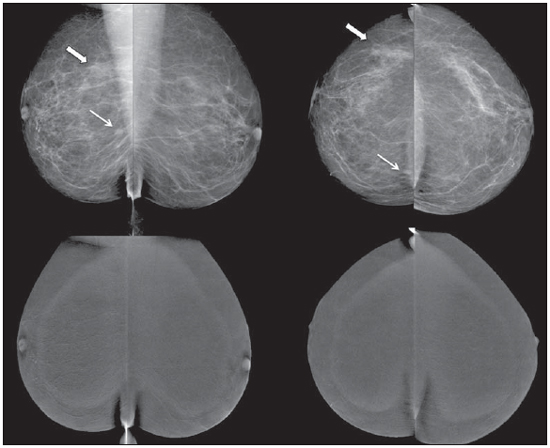 Figure 1. Contrast-enhanced digital mammography of a 65-year-old woman. The upper traditional digital mammographic images demonstrate density of focal asymmetry on the upper lateral quadrant (bold arrow) and presence of a nodule in the lower inner quadrant (thin arrow), both in the right breast. Lower recombined images (high-energy less low-energy) demonstrate absence of suspicious enhancement, suggesting benignity. 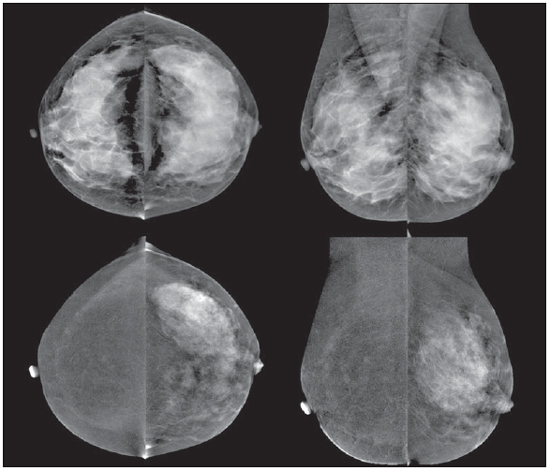 Figure 2. 50-year-old woman presenting a palpable mass in her left breast. At digital mammography (upper image), no relevant finding is observed. Recombined images (lower images) clearly demonstrate the presence of a mass in the upper lateral quadrant of the left breast. Biopsy demonstrated the presence of a ductal carcinoma in situ. MOLECULAR BREAST IMAGING The traditional breast scintigraphy has been utilized since the decade of the 1990's, when Tc-99 sestamibi tracer uptake by breast lesions was observed. Such a tracer is utilized in myocardial scintigraphy(24). This is a good method to evaluate both benign and malignant breast lesions, since its performance is not affected by breast density. Because of the large distance between the detector and the breast, this method presents low sensitivity for lesions < 10 mm and for those adjacent to the chest wall. New gamma camera dedicated to breast evaluation have been developed to improve the traditional scintigraphy resolution(25). Such apparatuses present a common characteristic - the positioning of detectors on parallel plates -, allowing images acquisition with positioning analogous to mammography and, as a result with increased sensitivity for detecting small-sized lesions. A breast-specific gamma camera was developed with high-resolution semiconductor detectors (CZT - cadmium zinc telluride) replacing the traditional photomul-tiplier tubes. Such detectors allow an increase in spatial resolution, with detection of lesions < 0.2 cm(26-29). Aiming at differentiating the method it was called molecular breast imaging (MBI), and the authors named it high-resolution breast scintigraphy, in an attempt to connect the traditional breast scintigraphy with the new high-resolution technology. The examination is performed with the woman seated and with the breasts immobilized with slight compression, about one third of the compression in mammography. The images may be acquired at any mam-mographic view. In order to facilitate the correlation with mammographic images, cranio-caudal and mediolateral-oblique views are included as a routine. In the literature, the images acquisition time is variable, ranging between 5 and 10 minutes(28,29). As the radiation dose is considered, there is a substantial difference between scintigraphy and mammography. At mammography, the radiation affects only the breasts, whereas at scintigraphy the whole body is involved with particular emphasis on the digestive and urinary systems. The technetium dose reported in the greatest majority of studies was 20-30 mCi, which corresponds to about 7 mSv (whole body dose), 2-7 times the radiation dose in a mammographic study (1-3 mSv). Studies have been developed with lower radiation doses (8 mCi and up to 4 mCi) in order to reach a final radiation dose similar to the dose at mammography(26). In patients with suspicious mammographic and sonographic findings the method presented a global sensitivity of 91% (97% for lesions > 10 mm, 91% for lesions measuring between 5 and 10 mm, and 69% for lesions < 5 mm)(30). Additionally, further lesions were found in 10% of the cases(31). As the method is compared with MRI, there was 97% agreement, with MRI sensitivity of 98% and 94% for MBI(30). Such a difference is principally due to lesions measuring between 0.2 and 0.4 cm(30). In the authors experience with the GE Discovery NM 750B system (GE Healthcare; Haifa, Israel), with an acquisition protocol of 10 minutes per view, contrast uptake was observed in ductal carcinomas in situ, invasive carcinomas, lobular carcinomas, phylloid tumors and fibroadenomas (Figures 3 and 4). At MRI studies of some women, the authors observed a similar sensitivity for lesions > 0.5 cm. 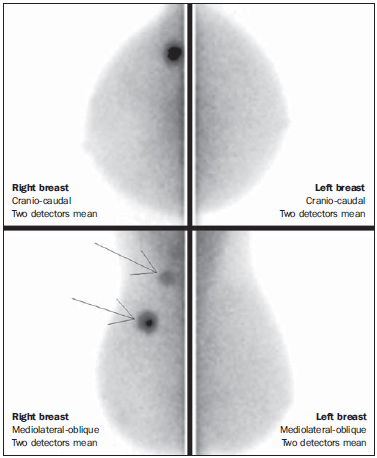 Figure 3. MBI study of a 59-year-old woman with dense breasts whose mammogram was inconclusive (BI-RADS 0). Strongly intense tracer uptake is observed in the upper lateral quadrant of her right breast with intense uptake by the ipsilateral axillary lymph node. Biopsy demonstrated the presence of an invasive ductal carcinoma with lymph node metastasis. 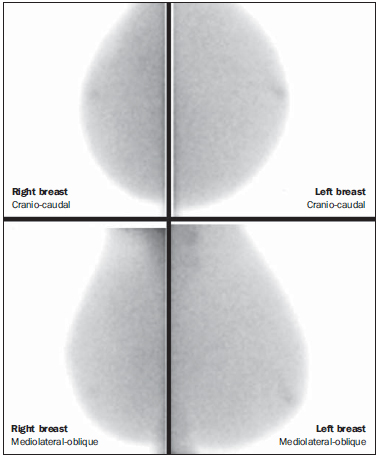 Figure 4. 55-year-old women submitted to conservative surgery in her right breast. At the MBI study, reduction in volume of the right breast is observed, but no suspicious tracer uptake is present, suggesting absence of residual or recurrent lesion. DISCUSSION Novel vascular/functional evaluation techniques for breast lesions have recently emerged. Their best clinical indications are still to be established but, theoretically they should be similar to the MRI clinical indications. At screening, such techniques might detect occult lesions in dense breasts, clearing up dubious findings (asymmetry or focal distortions, asymmetry seen on a single view); in the diagnosis, they would reduce the number of unnecessary biopsies; in the treatment planning, they would be useful in the evaluation of the local disease extent and in the detection of other foci; in both chemo and radiotherapy treatment follow-up, they would allow an evaluation of the treatment response. Contrast-enhanced mammography, as well as MRI, evaluates the morphology and vascularization of lesions. The major advantages in relation to MRI include a perfect correlation with mammographic images, lower cost and shorter acquisition time. Disadvantages include the current unfeasibility of performing biopsies and marking of enhancement areas, besides possible adverse effects of iodinated contrast agents. As compared with MRI, molecular breast imaging presents the following advantages: positioning analogous to mammography, facilitating less complex comparisons and interpretations, and lower cost. As compared with mammography, molecular breast imaging applies lower breast compression force and its sensitivity does not depend on the breast density. Disadvantages include longer acquisition time, a still high total radiation dose, and unfeasibility of morphological analysis. The false-positive results described in the literature and observed in the authors experience are similar to those at MRI, namely, fibroadenoma, adenosis, fibro-cystic changes, papilloma, phylloid tumor fibrosis, inflammatory fat necrosis, radial scar, focal inflammation and pseudoangio-matous hyperplasia(20,21,26,29). It is important to note that initial studies, the present one included, do not exactly represent the actual population. The observers involved in the images interpretation are more careful in their analyses and, in several studies, the findings are more valued, since their populations present a high index of cancer. As MRI is taken as a basis for a comparative cost analysis, molecular breast imaging costs about 70-80%, and contrast-enhanced mammography, 50% of the MRI cost. CONCLUSION Novel techniques for functional evaluation of breasts are currently available, presenting promising results and, in some cases, a performance similar to MRI. Their indications might be the same as for MRI, with the advantage of lower cost. Further results should be expected in order to define a procedure flowchart and thus making a good use of the advantages of each technology with minimum injury and risk for the population. REFERENCES 1. Brasil. Ministério da Saúde. Instituto Nacional de Câncer. Câncer: incidência de câncer no Brasil. Rio de Janeiro, RJ: INCA; 2012. 2. Miranda CMNR, Santos CJJ, Maranhão CPM, et al. A tomografia computadorizada multislice é uma ferramenta importante para o estadiamento e seguimento do câncer de mama? Radiol Bras. 2012;45:105-12. 3. Brennan ME, Houssami N. Evaluation of the evidence on staging imaging for detection of asymptomatic distant metastases in newly diagnosed breast cancer. Breast. 2012;21:112-23. 4. Chala LF, Barros N. Avaliação das mamas com métodos de imagem. Radiol Bras. 2007;40(1):iv-vi. 5. Boyd NF, Guo H, Martin LJ, et al. Mammographic density and the risk and detection of breast cancer. N Engl J Med. 2007;356:227-36. 6. Buist DSM, Porter PL, Lehman C, et al. Factors contributing to mammography failure in women aged 40-49 years. J Natl Cancer Inst. 2004;96:1432-40. 7. Gwin JL, Eisenberg BL, Hoffman JP, et al. Incidence of gross and microscopic carcinoma in specimens from patients with breast cancer after re-excision lumpectomy. Ann Surg. 1993;218:729-34. 8. Alvares BR, Freitas CHA, Jales RM, et al. Densidade mamográfica em mulheres menopausadas assintomáticas: correlação com dados clínicos e exames ultrassonográficos. Radiol Bras. 2012;45:149-54. 9. Prado GLM, Guerra MTPM. Valor preditivo positivo das categorias 3, 4 e 5 do Breast Imaging Reporting and Data System (BI-RADS®). Radiol Bras. 2010;43:171-4. 10. Calas MJG, Gutfilen B, Pereira WCA. CAD e mamografia: por que usar esta ferramenta? Radiol Bras. 2012;45:46-52. 11. Calas MJG, Alvarenga AV, Gutfilen B, et al. Avaliação de parâmetros morfométricos calculados a partir do contorno de lesões de mama em ultras-sonografias na distinção das categorias do sistema BI-RADS. Radiol Bras. 2011;44:289-96. 12. Marques EF, Medeiros MLL, Souza JA, et al. Indicações de ressonância magnética das mamas em um centro de referência em oncologia. Radiol Bras. 2011;44:363-6. 13. Pereira FPA, Martins G Figueiredo E, et al. O uso da difusão por ressonância magnética na diferenciação das lesões mamárias benignas e malignas. Radiol Bras. 2009;42:283-8. 14. Kuhl C. The current status of breast MR imaging. Part I. Choice of technique, image interpretation, diagnostic accuracy, and transfer to clinical practice. Radiology. 2007;244:356-78. 15. Kuhl CK. Current status of breast MR imaging. Part 2. Clinical applications. Radiology. 2007; 244:672-91. 16. Yeh ED. Breast magnetic resonance imaging: current clinical indications. Obstet Gynecol Clin North Am. 2011;38:159-77, ix. 17. Mameri CTS. Impacto da ressonância magnética mamária no tratamento cirúrgico, abordagem axilar e terapêutica sistêmica do câncer de mama. Radiol Bras. 2008;41:422. 18. Dromain C, Balleyguier C, Adler G, et al. Contrast-enhanced digital mammography. Eur J Radiol. 2009;69:34-42. 19. Helvie MA. Digital mammography imaging: breast tomosynthesis and advanced applications. Radiol Clin North Am. 2010;48:917-29. 20. Dromain C, Thibault F, Muller S, et al. Dual-energy contrast-enhanced digital mammography: initial clinical results. Eur Radiol. 2011;21:565-74. 21. Lewin JM, Isaacs PK, Vance V, et al. Dual-energy contrast-enhanced digital subtraction mammography: feasibility. Radiology. 2003;229:261-8. 22. Jong RA, Yaffe MJ, Skarpathiotakis M, et al. Contrast-enhanced digital mammography: initial clinical experience. Radiology. 2003;228:842-50. 23. Diekmann F, Freyer M, Diekmann S, et al. Evaluation of contrast-enhanced digital mammography. Eur J Radiol. 2011;78:112-21. 24. Khalkhali I, Vargas HI. The role of nuclear medicine in breast cancer detection: functional breast imaging. Radiol Clin North Am. 2001;39:1053-68. 25. Majewski S, Curran E, Keppel C, et al. Optimization of dedicated scintimammography procedure using detector prototypes and compressible phantoms. Nuclear Science Symposium Conference Record, 2000 IEEE; 2000. 26. Rhodes DJ, Hruska CB, Phillips SW, et al. Dedicated dual-head gamma imaging for breast cancer screening in women with mammographically dense breasts. Radiology. 2011;258:106-18. 27. Tafreshi NK, Kumar V, Morse DL, et al. Molecular and functional imaging of breast cancer. Cancer Control. 2010;17:143-55. 28. Hruska CB, Rhodes DJ, Collins DA, et al. Evaluation of molecular breast imaging in women undergoing myocardial perfusion imaging with Tc-99m sestamibi. J Womens Health (Larchmt). 2012;21:730-8. 29. Kim BS. Usefulness of breast-specific gamma imaging as an adjunct modality in breast cancer patients with dense breast: a comparative study with MRI. Ann Nucl Med. 2012;26:131-7. 30. Hruska C, Boughey J, Phillips S, et al. Molecular breast imaging: a review of the Mayo Clinic experience. Am J Surg. 2008;196:470-6. 31. Rhodes DJ, O'Connor MK, Phillips SW, et al. Molecular breast imaging: a new technique using technetium Tc 99m scintimammography to detect small tumors of the breast. Mayo Clin Proc. 2005;80:24-30. 1. Master, MD, Radiologist, IMEB - Imagens Médicas de Brasília, Brasília, DF Brazil. 2. Fellow Master degree, Nuclear Physician, IMEB - Imagens Médicas de Brasília, Brasília, DF Brazil. 3. Nuclear Physician, Technical Director, IMEB - Imagens Médicas de Brasília, Brasília, DF, Brazil. Mailing Address: Dr. Filipe Ramos Barra SMHN, Quadra 2, Bloco C, Ed. Dr. Crispim, Sobreloja 18, IMEB Brasília, DF, Brazil, 70710-100 E-mail: filipe@imeb.com.br Received May 20, 2012. Accepted after revision September 25, 2012. * Study developed at IMEB - Imagens Médicas de Brasília, Brasília, DF, Brazil. |
|
Av. Paulista, 37 - 7° andar - Conj. 71 - CEP 01311-902 - São Paulo - SP - Brazil - Phone: (11) 3372-4544 - Fax: (11) 3372-4554