INTRODUCTION
Infantile myofibromatosis, a rare childhood disease, was first described by Stout in 1954, as congenital generalized fibromatosis(1). Classically, this disease presents solitary or multicentric nodular masses arising at the first months of life and involving the skin, subcutaneous tissues, bones or viscus(1–3).
The present report is aimed at reviewing the findings of this rare disease, emphasizing that the diagnosis must be sped up considering the significant morbidity of this condition.
CASE REPORT
A male, three-month-old patient presented with incoercible vomiting since his ninth day of life, evolving with significant weight loss and progressive development of skin nodules, particularly on the scalp and trunk (Figure 1A).
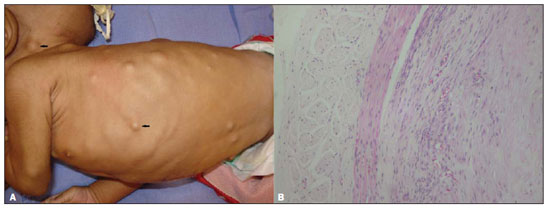
Figure 1. Skin lesions.
A: Innumerable subcutaneous nodules on the trunk and scalp.
B: Biopsy slide with visceral nodule specimen demonstrating muscle cells and fibroblasts.
Investigation of upper intestinal obstruction was performed with contrast-enhanced imaging of the esophagus, stomach and duodenum, demonstrating an apparently extrinsic narrowing in the pyloric region. The patient was submitted to exploratory laparotomy that demonstrated the presence of a mass adjacent to the pylorus which was biopsied. Histopathological study revealed spindle cells characteristic of muscle cells, intermingled with fibroblasts, confirming the diagnosis of infantile myofibromatosis (Figure 1B).
The lesions workup proceeded with ultrasonography which demonstrated the presence of diffuse, hypoechoic nodules with a more echogenic nucleus affecting the subcutaneous tissue of the trunk, scalp (Figure 2A) and the gallbladder wall (Figure 2B). Computed tomography demonstrated subtly hypodense lesions with homogeneous contrast uptake on the late phase (Figure 3). Magnetic resonance imaging (MRI) demonstrated the presence of nodules in subcutaneous, intramuscular and visceral tissues, with hyposignal on T1- weighted sequences (Figure 4A), variable hypersignal on T2-weighted sequences (Figure 4B) and peripheral paramagnetic contrast uptake (Figure 4C).
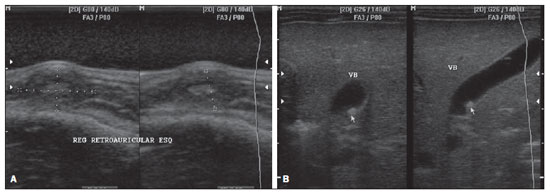
Figure 2. Ultrasonography.
A: Hypoechoic nodules in subcutaneous tissue with hyperechoic nucleus, causing skin bulging.
B: Image of parietal nodule in the gallbladder with iso- to hyperechoic aspect.
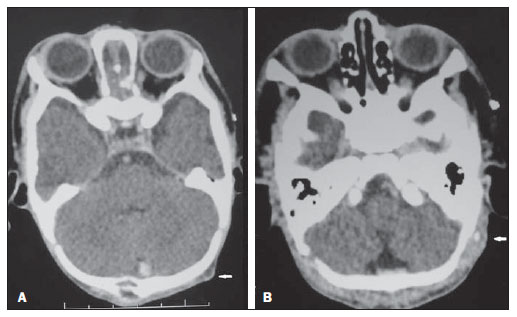
Figure 3. Computed tomography.
A: Hypodense nodule in the left occipital region.
B: Appearance of late contrast enhancement on the central region of nodules.
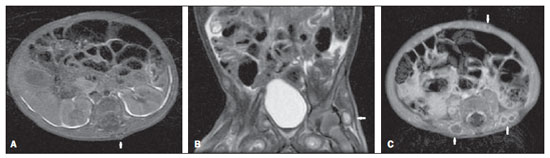
Figure 4. Magnetic resonance imaging.
A: Axial T1-weighted image demonstrates dorsal nodule with iso/hyposignal.
B: Coronal T2-weightd image with fat saturation demonstrating deep nodule with marked hypersignal.
C: Axial T1-weighted image following gadolinium injection, demonstrating peripheral enhancement of subcutaneous nodules.
The pathogenesis of generalized fibromatosis still remains unknown, despite reports on association with estrogen receptors(4). This disease is classified into three types as follows: solitary fibromatosis, congenital generalized fibromatosis without visceral involvement, and congenital generalized fibromatosis with both cutaneous and visceral involvement(5). In most cases, the disease is sporadic, but there are reports on familial occurrence of the three presentations of the disease, so a possible genetic autosomal inheritance (either dominant or recessive) is postulated, with a male prevalence(3). The range of differential diagnoses is wide and should include other types of fibromatosis, congenital infantile fibrosarcoma, hemangiopericitoma, myofibroblastic tumors, neurofibromas, leyomiomas and nodular fasciitis(6,7).
The imaging findings of the present case are compatible with those described by other reports in the literature, but one should consider the extent of the lesion, with possible central degeneration in cases of major masses. MRI imposes itself as the best method to evaluate the extent and visceral involvement by the disease.
The treatment still remains controversial and includes surgery in cases of obstruction like in the present report, radiotherapy, corticotherapy and chemotherapy – all of these treatments still with limited results(6). The disease prognosis is less favorable in cases of visceral involvement(3). The patient is currently undergoing chemotherapy and has presented a significant clinical and radiological improvement of the lesions and good weight gain progression. The relevance of imaging methods in the identification of lesions which otherwise could not be identified at physical examination, as well as their relationship with anatomical structures, should be highlighted.
Therefore, the association of imaging methods and histopathological analysis allows the staging and classification of the disease, leading to the adoption of the most appropriate treatment for this rare pathological entity. The authors conclude that the knowledge of the typical radiological findings of this entity is critical, considering the wide range of differential diagnoses including disorders commonly found in children.
REFERENCES
1. Stout AP. Juvenile fibromatosis. Cancer. 1954;7:953–78.
2. Dimson OG, Drolet BA, Southern JF, et al. Congenital generalized myofibromatosis in a neonate. Arch Dermatol. 2000;136:597–600.
3. Robbin MR, Murphey MD, Temple T, et al. Imaging of musculoskeletal fibromatosis. Radiographics. 2001;21:585–600.
4. Chapman PR, Judd CD, Felgenhauer JL, et al. Infantile myofibromatosis of the posterior fossa. AJR Am J Roentgenol. 2005;184:1310–2.
5. Thunnissen BT, Bax NM, Rövekamp MH, et al. Infantile myofibromatosis: an unusual presentation and a review of the literature. Eur J Pediatr Surg. 1993;3:179–81.
6. Kuo FY, Huang SC, Eng HL, et al. Solitary infantile myofibromatosis: report of two cases. Chang Gung Med J. 2002;25:393–8.
7. Schmidt D. Fibrous tumors and tumor-like lesions of childhood: diagnosis, differential diagnosis, and prognosis. Curr Top Pathol. 1995;89:175–91.
1. MDs, Residents in Radiology and Imaging Diagnosis, Universidade Federal do Espírito Santo (UFES), Vitória, ES, Brazil.
2. Radiologist, Physician Assistant, Hospital Infantil Nossa Senhora da Glória, Vitória, ES, Brazil.
Mailing Address:
Dra. Bruna Emmanuelle Linhares Fonseca Mata
Avenida Rio Branco, 1512, ap. 1003, Praia do Canto
Vitória, ES, Brazil, 29055-642
E-mail: brunaemmanuelle@yahoo.com.br
Received September 1st, 2011.
Accepted after revision November 10, 2011.
Study developed at Hospital Infantil Nossa Senhora da Glória and Hospital Universitário Cassiano Antônio de Moraes, Vitória, ES, Brazil.
 Vol. 45 nº 2 - Mar. / Apr. of 2012
Vol. 45 nº 2 - Mar. / Apr. of 2012



