INTRODUCTION
In the last years, expressive developments in the field of radiology and imaging diagnosis have been observed, with the advent of multidetector computed tomography (MDCT) with up to 320 channels and high-field magnetic resonance imaging (MRI) (3 T), among other imaging methods, with the consequential acquisition of images with higher quality and accuracy. In the context of such evolution, and in an attempt to obtain not only a structural assessment, but also a metabolic and functional analysis of different organs and types of lesions, new imaging techniques have been developed. Amongst these new imaging modalities, diffusion MRI, magnetic resonance spectroscopy, positron emission tomography (PET-CT), and perfusion CT and MRI can provide not only structural, but also functional data. Data provided by such group of imaging methods have been called "biomarkers", since they allow the analysis of the biological behavior of healthy and diseased tissues, amongst other utilities allowing an early prediction of the therapeutic response to chemotherapy treatments, particularly those utilizing drugs with cytostatic and antiangiogenic properties(1).
The range of indications for such imaging methods is quite varied, including differentiation between inflammatory process and tumor-like lesions, determination of the degree of functional compromising of certain organs, detection of the presence of residual or recidivating tumor tissue after minimally invasive therapies, among others(1—7). Amongst the mentioned tools, perfusion CT (PCT) has recently aroused the interest of many researchers(1,3,5,7), given its reproducibility, robustness and efficacy resulting from the current wide availability of CT apparatuses equipped with the multidetector technology (MDCT) in the public health system as well as in the authors' institution.
METHOD APPLICATION
PCT is a relatively recent technique that allows a functional analysis of tissues by evaluating their vascularization. Such method evaluates temporal alterations in the tissue density following intravenous contrast injection, by acquisition of a dynamic image series(1).
Thus, this method has initially played a key role in the evaluation of patients with acute ischemic cerebrovascular insult, assisting in the therapeutic and, consequently, influencing the evaluation of the patient's prognosis(8).
In the last years, the utilization of this method for abdominal radiology has increased, particularly in the field of oncology where PCT has been utilized in the diagnosis, staging, prognostic evaluation and therapeutic response monitoring, with potential for being the method of choice in the evaluation of the tumor response to antiangiogenic drugs as well as to minimally invasive treatments such as intra-arterial embolization, percutaneous alcoholization and radiofrequency thermoablation(1,6,9,10).
Some studies have also demonstrated other utilities of PCT in abdominal radiology, including differentiation between diverticulitis and colorectal neoplasia(7), evaluation of perfusion alterations in hepatic cirrhosis(2,4,5,11) and follow-up of patients submitted to organ transplantation(12).
Some results of such studies as those observed in the differentiation between diverticulitis and colorectal cancer(7) and in the evaluation of patients with severe acute pancreatitis(13) have shown to be quite interesting. In cases of acute pancreatitis, PCT has shown 100% sensitivity and 95% specificity in the detection of pancreatic ischemia, which may assist in the prediction of later development of pancreatic necrosis(13). It is known that pancreatic necrosis increases substantially the morbimortality, and the early detection of such a condition allows a more intensive treatment, preventing other complications, thus improving the patient's prognosis(13). Also, in the distinction between diverticulitis and colorectal cancer, PCT has revealed to be useful for allowing the identification of a significant difference in the main perfusion parameters between patients with colorectal cancer and patients with diverticulitis. In this sense, 80% sensitivity and 75% specificity were observed respectively for blood volume (BV) and blood flow (BF) in the diagnosis of cancer(7).
IMAGING TECHNIQUE: BASIC PRINCIPLES
The study and analysis by PCT are based on temporal alterations of tissue attenuation measured in Hounsfield units (HU) following intravenous contrast injection. The tissue enhancement depends on the iodine concentration, and indirectly reflects the tissue vascularization and the vascular physiology(14,15). After iodinated contrast medium injection, the tissue enhancement may be divided into two phases according to the contrast agent distribution in the intra- and extra-vascular compartments(14).
At the early phase, the enhancement is purely attributed to the contrast agent distribution within the intravascular space, and such a phase usually lasts 40 to 60 seconds after contrast uptake into this compartment(1,2,14—16). At the second phase, the contrast agent passes through the basal membrane, from the intravascular into the extravascular compartment. Thus, the tissue enhancement results from the contrast agent distribution between these two compartments(1,14).
At the early phase, the enhancement is mostly determined by the tissue blood flow and by the blood volume, while at the second phase it is most influenced by the vascular permeability(1).
Blood flow or perfusion is defined as the blood flow through the tissue of interest per unit of time and volume, measured in milliliters of blood per 100 grams of tissue per minute (ml/100 g/min). Blood volume is defined as the blood volume passing through the region of interest, measured in milliliters of blood per 100 grams of tissue (ml/100 g). Peak enhancement (PE) represents the peak tissue enhancement resulting from the contrast agent injection and measured in HU(10).
Time to peak (TTP) is defined as the elapsed time before the peak enhancement is achieved, and is measured in seconds. On the other hand, mean transit time (MTT) represents the mean time it takes for blood to circulate through capillaries of a determined region, passing from arterioles to venules, being measured in seconds. Permeability surface (PS) is related to the coefficient of contrast molecules diffusion through the capillary endothelium pores to the extravascular space, and is measured in ml/100 g/min(10).
Thus, the currently available tomographic apparatuses allow a rapid and successive images acquisition covering a region of interest during both phases, and capturing temporal alterations in tissue attenuation, applying appropriate mathematical models to such images in order to quantify the tissue perfusion(1,2,14).
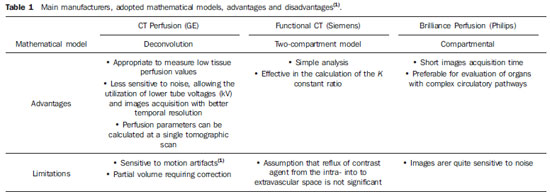
Compartmental analysis and deconvolution represent the two most frequently utilized mathematical models for data processing, which in a certain way will affect the imaging protocol design(1,2).
Mathematical models
a) Compartmental analysis
In this model, the analysis can be performed either by the one-compartment model or by the two-compartment model(2,10,16).
The one-compartment model assumes both the intravascular and extravascular spaces as a single compartment, and the calculation of the tissue perfusion is based on the principle of conservation of mass in a system (Fick principle). According to this principle, perfusion is calculated from the maximal slope of the tissue concentration-time curve normalized to the curve of arterial concentration of contrast agent (artery input function)(2,10,15).
The two-compartment model assumes the intravascular and extravascular spaces as distinct compartments(1,2). Such a model utilizes the technique known as Patlak analysis that quantifies the flow from the intravascular into the extravascular compartment and measures the capillary permeability and the blood volume(2,10,14—16).
b) Deconvolution
This model is based on the utilization of arterial and tissue time-concentration curves to calculate the impulse residue function (IRF) for the tissue of interest(1,2), where IRF corresponds to a theoretical tissue curve obtained from data regarding direct arterial input. The height of the curve reflects the tissue perfusion, and the area under the curve reflects the blood volume(2).
Preliminary studies show a strong correlation between results obtained by these two models, suggesting equivalence between them(2). However, there are differences regarding validation and susceptibility to motion and noise(2,17).
Compartmental analysis is based on the hypothesis that the contrast agent bolus must be retained in the organ of interest at the moment of measurement, which may result in underestimation of perfusion values in organs with rapid vascular transit(1,2,18).
Deconvolution, however, assumes that the shape of the IRF is a plateau with a single exponential washout. Although such a hypothesis is validated for most of the organs, it might not be appropriate for organs with complex circulatory pathways(1,2,18).
Thus, the compartmental model is preferable to evaluate organs with complex circulatory pathways such as the kidneys and spleen(1). Also, it is quite useful in the evaluation of the liver, since it provides a separate estimation of the arterial and portal components(19—23).
On the other hand, deconvolution is appropriate to measure low tissue perfusion values (< 20 ml/min/100 ml), typically observed in post-treatment tumors(1,2,19,20).
Deconvolution is less sensitive to noise, allowing the utilization of lower tube voltage (kV) and images acquisition with better temporal resolution. In the compartmental model, the presence of noise on images might result in errors in the perfusion values calculation(1). Several manufacturers have adopted different PCT calculation models in their apparatuses (Table 1).
Imaging protocol
Several PCT protocols have been proposed, depending on different variables such as organ of interest, mathematical model utilized (compartmental or deconvolution), available equipment and clinical objective. Amongst such variables, the mathematical model and the equipment have direct influence on the imaging protocol (for example: contrast agent volume, injection rate and interval between contrast injection initiation and data acquisition or delay) and on technical parameters (kV and mAs). Thus, each one of such variables is detailed below.
Typically, PCT consists of a non-contrast-enhanced phase followed by a contrast-enhanced phase where dynamic images of the region of interest are acquired. The dynamic images acquisition may include the study of the first contrast pass, a delayed study, or both, according to the parameters to be evaluated(2).
a) Non-contrast-enhanced phase
This is the first phase of the study, where a scan is performed from the lung bases to the pubic symphysis, utilizing the technical parameters habitually adopted by each unit. Such phase is primarily aimed at identifying and delineating the region of interest (or coverage area) that will be analyzed after the contrast injection. The coverage area will depend on the CT equipment utilized. Coverage area in 4 or 16-channel equipment corresponds to up to 2 cm; and in 64-channel equipment, to 4 cm. Larger coverage areas (8 to 16 cm) can be obtained with 128 and 320-channel equipment with several reconstruction algorithms(1).
b) Dynamic (post-contrast or contrast-enhanced) phase
This phase is the most important in the study, since it provides the necessary data for processing and perfusion analysis. In such a phase, images of the previously delimited area are dynamically acquired after contrast agent injection.
For a first pass study with deconvolution, images are acquired every second; with the compartmental model, images are acquired at every 3—5 seconds, during the first 40—60 seconds(2,4,7).
With this dynamic images acquisition, it is possible to observe the arrival of the contrast agent and its first pass through the intravascular space(1).
Some technical parameters, such as mAs, kVp, contrast agent volume and concentration and injection rate, are extremely important for this phase, since they have a direct influence on the study results. The literature presents great variation in such parameters, which depend on the CT equipment (manufacturer and number of detector rows) and on the mathematical model adopted for data analysis. The following topics include comments on each of these parameters.
b.1) Technical parameters (mAs and kVp)
The utilization of low tube voltage (kVp) and current (mAs) is recommended to reduce the radiation dose delivered to the patient. A tube voltage between 80 and 100 kVp is generally appropriate for most clinical applications of PCT(1,2,10).
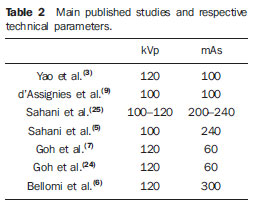
The literature review demonstrates a great variation in mAs values (from 50 to 250 mAs), depending on the adopted mathematical model. For deconvolution, the adoption of values between 50 and 100 mAs is recommended. The compartmental model requires higher mAs values (100 to 250 mAs)(1,5,24) (Table 2).
b.2) Radiation dose
The effective radiation dose utilized in some studies ranged from 10 to 12 mSv(7,9,24), depending on the adopted technical parameters.
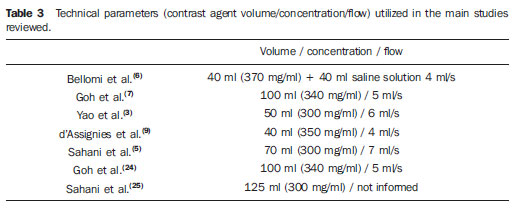
In the authors' experience, the PCT technique has added, on average, about 12 mSv/patient at conventional CT, whose radiation dose for the three phases has ranged from 20 to 25 mSv (between 7 and 8 mSv per acquisition phase). Such values are within the limits recommended for yearly ionizing radiation exposure(25). Several studies have sought to demonstrate the value of PCT obtained with low radiation doses by means of kVp(5,9) and mAs(7,24) reduction (Table 3).
b.3) Contrast agent volume, concentration and injection rate
A contrast agent volume between 40 and 70 ml and an injection rate ranging between 3.5 and 10 ml/s are generally appropriate for an optimum perfusion analysis(1,2,10,16).
Such wide variation is a function of the mathematical model adopted for each equipment. An injection rate between 5 and 10 ml/s is preferable for those utilizing the compartmental model. Lower injection rates (for example, 3.0 to 5.0 ml/s) are utilized for those with deconvolution. However, as already mentioned, high injection rates (7 to 10 ml/s) are considered as beneficial to maximize to tissue enhancement and the signal-noise ratio (Table 3)(1). In the authors' studies, an injection rate corresponding to 6.0 ml/s has been adopted, with a 18-20G Gelco catheter inserted into a large antecubital vein.
Because of the linear relations between the iodine concentration and the tissue enhancement, higher iodine concentrations (370 to 400 mg/ml) in the contrast agent are considered as ideal(1,2,16) and, whenever possible, such a concentration has been adopted by the authors.
b.4) Interval (delay)
In most clinical applications, a five to 15-second delay between contrast injection and the initiation of images acquisition is considered as appropriate, depending on the region to be evaluated, a delay between five and eight seconds being ideal for studies in the cervical, thoracic and abdominal regions, and between 10 and 15 seconds for studies of pelvis and extremities(1).
CT images acquisition
In the authors' institution, abdominal and pelvic CT studies as performed in a Philips Brilliance 64-detector row, multislice equipment (Philips Medical Systems; Best, Netherland) with a dedicated perfusion software (Brilliance Perfusion 4.0).
As already mentioned, the PCT study is performed in two phases.
The first phase comprises volumetric, non-contrast-enhanced images acquisition, from the diaphragm to the pubic symphysis, with the multislice technique. The technical CT parameters utilized in this phase involve collimation = 64 × 0.625 mm, 120 kVp, mAs 120, pitch = 0.891 and 3 mm slice thickness. This first phase is aimed at delineating the region of interest, with 4 cm in extent, where the perfusion analysis will be performed. New, still not widely available softwares for images acquisition and reconstruction (for example: Jog-scanr) allow doubling the extent of the area to be studied along the axis
z.
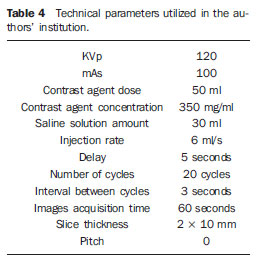
The second phase, corresponding to the profusion analysis itself, is performed after intravenous hydrosoluble, non-ionic iodinated contrast injection, with axial sections of the previously delimited area, according to the tomographic parameters described on Table 4, as previously published(3).
In order to reduce the presence of respiratory motion artifacts, that is the main cause for unsuccessful images acquisition, an abdominal compression belt (that is part of the equipment itself) is utilized, and the patient is instructed to breath as slowly and pausedly as possible to guarantee the acquisition of high-quality images, without respiratory artifacts (Figure 1).
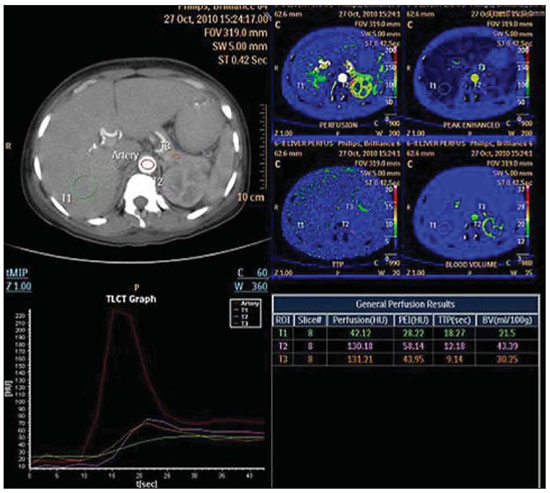
Figure 1. Perfusion computed tomography providing perfusion parameters of liver (VI segment), renal cortex and pancreas, as maps, graphics and absolute values. Free breathing imaging. No expressive respiratory artifact is observed on the images.
Once the PCT scan is finished, the acquired and reconstructed images are transferred to a workstation and processed with the aid of the CT perfusion software, with generation of color functional maps and calculation of functional parameters.
The software utilized is easily and automatically ran, and does not require user interference in the generation of functional maps.
Initially, an evaluation of the technical quality of the study must be performed to confirm the absence of respiratory and motion artifacts on the images. After that, a region of interest (ROI) must be drawn in the central region of a calibrous arterial vessel (e.g., the aorta) to be used as a reference in the evaluation of arterial contrast agent concentration in time (artery input function) (Figure 2). In studies of the pelvis, the iliac artery can be utilized as a reference vessel for evaluation of the arterial flow.
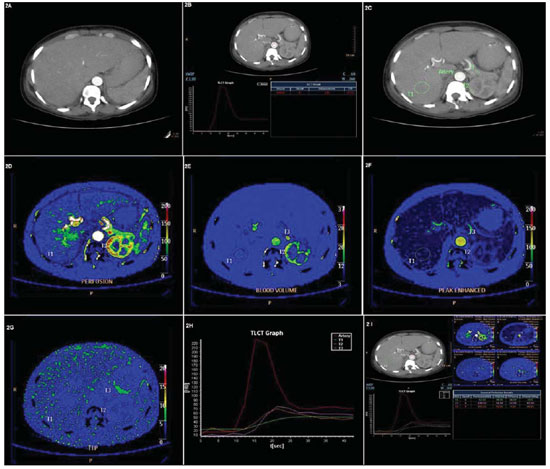
Figure 2. Perfusion computed tomography study. A step-by-step demonstration of the images processing. A: Step 1 involves the selection of dynamic/contrastenhanced images of the region of interest. B: Step II consists of drawing of a ROI on the aorta to be utilized as a reference for determining a curve of arterial contrast agent concentration in time (artery input function). C: Step III – ROIs are drawn on areas to be studies; in the present case, they were randomly distributed in the liver, renal cortex and pancreas. D,E,F,G,H: Subsequently (Step IV), perfusion parameters (P, BV, PE and TTP) are automatically represented as color maps, graphics and absolute values. I: PCT study overview with all the perfusion parameters on different presentations (color maps, graphics and absolute values).
Then, ROIs are freehand drawn around the lesions/regions of interest, coinciding exactly with their outlines for quantification of functional parameters (Figure 2). Once ROIs are defined on the lesions to be studies, the software CT perfusion automatically calculates the perfusion values according to the equipment and mathematical model utilized(1).
CONCLUSION
The range of indications for PCT is quite varied, including differentiation between inflammatory process and tumor-like lesions, determination of the degree of functional compromising of certain organs, detection of the presence of residual or recidivating tumor tissue after minimally invasive therapies, among others.
The main difference of PCT as compared with other imaging techniques is related to its capability of characterizing distinct perfusion behaviors translating into biological alterations resulting from certain lesions and diseased tissues.
After a comprehensive literature review and adoption of PCT in the authors' clinical routine, it may be concluded that this method is easy to perform, with applicability in cases of several abdominal conditions. Thus, it is the authors' opinion that such a method will be useful not only in the field of research, but also in the daily practice as a valuable tool in addition to conventional computed tomography.
However, strategies aimed at radiation doses reduction and softwares to allow an increase in the evaluation extent may further enhance the utilization of the method.
REFERENCES
1. Kambadakone AR, Sahani DV. Body perfusion CT: technique, clinical applications, and advances. Radiol Clin North Am. 2009;47:161—78.
2. Miles KA. Perfusion CT for the assessment of tumour vascularity: which protocol? Br J Radiol. 2003;76 Spec No 1:S36—42.
3. Yao J, Yang ZG, Chen TW, et al. Perfusion changes in gastric adenocarcinoma: evaluation with 64-section MDCT. Abdom Imaging. 2010;35:195—202.
4. Pandharipande PV, Krinsky GA, Rusinek H, et al. Perfusion imaging of the liver: current challenges and future goals. Radioloy. 2005;234:661—73.
5. Sahani DV, Holalkere NS, Mueller PR, et al. Advanced hepatocellular carcinoma: CT perfusion of liver and tumor tissue — initial experience. Radiology. 2007;243:736—43.
6. Bellomi M, Petralia G, Sonzogni A, et al. CT perfusion for the monitoring of neoadjuvant chemotherapy and radiation therapy in rectal carcinoma: initial experience. Radiology. 2007;244:486—93.
7. Goh V, Halligan S, Taylor SA, et al. Differentiation between diverticulitis and colorectal cancer: quantitative CT perfusion measurements
versus morphologic criteria — initial experience. Radiology. 2007;242:456—62.
8. Koenig M, Klotz E, Luka B, et al. Perfusion CT of the brain: diagnostic approach for early detection of ischemic stroke. Radiology. 1998;209:85—93.
9. d'Assignies G, Courvelard A, Bahrami S, et al. Pancreatic endocrine tumors: tumor blood flow assessed with perfusion CT reflects angiogenesis and correlates with prognostic factors. Radiology. 2009;250:407—16.
10. Miles KA, Charnsangavej C, Lee FT, et al. Application of CT in the investigation of angiogenesis in oncology. Acad Radiol. 2000;7:840—50.
11. Van Beers BE, Leconte I, Materne R, et al. Hepatic perfusion parameters in chronic liver disease: dynamic CT measurements correlated with disease severity. AJR Am J Roentgenol. 2001;176:667—73.
12. Dawson P. Functional imaging in CT. Eur J Radiol. 2006;60:331—40.
13. Tsuji Y, Yamamoto H, Yazumi S, et al. Perfusion computerized tomography can predict pancreatic necrosis in early stages of severe acute pancreatitis. Clin Gastroenterol Hepatol. 2007;5:1484—92.
14. Miles KA. Tumour angiogenesis and its relation to contrast enhancement on computed tomography: a review. Eur J Radiol. 1999;30:198—205.
15. Miles KA, Griffiths MR. Perfusion CT: a worthwhile enhancement? Br J Radiol. 2003;76:220—31.
16. Miles KA. Functional computed tomography in oncology. Eur J Cancer. 2002;38:2079—84.
17. Cao J, Yang A, Long XY, et al. CT hepatic volume measurement combined with CT perfusion imaging in evaluating the hepatic functional reserve. Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2007;32:422—6.
18. Miles KA, McPherson SJ, Hayball MP. Transient splenic inhomogeneity with contrast-enhanced CT: mechanism and effect of liver disease. Radiology. 1995;194:91—5.
19. Miles KA, Hayball MP, Dixon AK. Functional images of hepatic perfusion obtained with dynamic CT. Radiology. 1993;188:405—11.
20. Blomley MJ, Coulden R, Dawson P, et al. Liver perfusion studied with ultrafast CT. J Comput Assist Tomogr. 1995;19:424—33.
21. Tsushima Y, Blomley JK, Kusano S, et al. The portal component of hepatic perfusion measured by dynamic CT: an indicator of hepatic parenchymal damage. Dig Dis Sci. 1999;44:1632—8.
22. Bader TR, Herneth AM, Blaicher W, et al. Hepatic perfusion after liver transplantation: noninvasive measurement with dynamic single-section CT. Radiology. 1998;209:129—34.
23. Miles KA, Leggett DA, Bennett GA. CT derived Patlak images of human kidney. Br J Radiol. 1999;72:153—8.
24. Goh V, Halligan S, Daley F, et al. Colorectal tumor vascularity: quantitative assessment with multidetector CT — do tumor perfusion measurements reflect angiogenesis? Radiology. 2008;249:510—7.
25. D'Ippolito G, Medeiros RB. Exames radiológicos na gestação. Radiol Bras. 2005;38:447—50.
1. MD, Abdominal Radiology Trainee, Department of Imaging Diagnosis, Universidade Federal de São Paulo (Unifesp), São Paulo, SP, Brazil.
2. MD, Radiologist, Unit of Abdomen, Department of Imaging Diagnosis, Universidade Federal de São Paulo (Unifesp), São Paulo, SP, Brazil.
3. MD, Collaborator for the Unit of Abdomen, Department of Imaging Diagnosis, Universidade Federal de São Paulo (Unifesp), São Paulo, SP, Brazil.
4. MD, Fellow PhD degree, Department of Imaging Diagnosis, Universidade Federal de São Paulo (Unifesp), São Paulo, SP, Brazil.
5. Professor, Private Docent, Department of Imaging Diagnosis, Universidade Federal de São Paulo (Unifesp), São Paulo, SP, Brazil.
Mailing Address:
Dr. Giuseppe D'Ippolito
Rua Professor Filadelfo Azevedo, 617, ap. 61, Vila Nova Conceição
São Paulo, SP, Brazil, 04508-011
E-mail: giuseppe_dr@uol.com.br
Received September 13, 2011.
Accepted after revision October 10, 2011.
Study developed at Department of Imaging Diagnosis, Universidade Federal de São Paulo (Unifesp), São Paulo, SP, Brazil.
 Vol. 45 nº 1 - Jan. /Feb. of 2012
Vol. 45 nº 1 - Jan. /Feb. of 2012