INTRODUCTION
Post-transplantation lymphoproliferative disorder (PTLD) is considered as a potentially fatal complication secondary to pharmacological immunosuppression in patients submitted to solid organs transplant. The incidence of PTLD varies according to the transplanted organ and is significantly higher in children(1—3), representing a wide spectrum of alterations characterized by different degrees of abnormal proliferation of lymphoid tissue which differ from each other in terms of pathogenesis, histological appearance and clinical behavior(1—4).
Most frequently, clinical manifestations of PTLD occur within the first year after transplantation and may affect any organ in the body, either focally or diffusely(2—4). The spectrum of such disorder includes hyperplastic lesions, polymorphic or monomorphic disease, the latter including lymphomas which represent the most severe form of involvement(5).
Imaging findings also vary, typically reflecting the possibility of involvement of multiple organs and systems. Besides suspecting the diagnosis on the basis of imaging findings, the radiologist plays a relevant role in the collection of material for histopathological analysis by means of image-guided percutaneous biopsy and in the evaluation of the treatment response(4,6,7).
Despite the fact that some studies have already evaluated the incidence and clinical presentation of PTLD(8—11), few authors have approached the imaging findings associated with this disease. The present study was aimed at evaluating the incidence and imaging findings of lymphomas after liver transplantation in children.
MATERIALS AND METHODS
After approval by the Committee for Ethics in Research of the institution, clinical records of all the children submitted to liver transplantation between 2000 and 2008 at Hospital A. C. Camargo, São Paulo, SP, Brazil, were retrospectively reviewed.
A standard questionnaire was fulfilled for all the children participating in the study, including the following variables: sex, age, transplant date, type of donor (deceased or living) liver disease that has led to transplant, type of immunosuppressant treatment, and post-transplant follow-up time. Another form was also fulfilled for patients who developed lymphoma along the postoperative follow-up, with the following data: time elapsed between transplantation and the diagnosis of lymphoma, clinical presentation, histological type of the tumor, sites of involvement, imaging findings and clinical outcome.
Imaging studies reports in the form of hard copies and digital files were retrieved from the archives of the Department of Imaging Diagnosis and included ultrasonography (US), computed tomography (CT), magnetic resonance imaging (MRI) and positron emission computed tomography (PET-CT). Some images were selected to illustrate cases of post-transplant lymphoma.
The statistical analysis was performed by the software SPSS for Windows, version 17.0 (SPSS Inc.; Chicago, IL, USA). Categorical data were presented in terms of percentages. Continuous variables were expressed as means and standard deviation in cases of normal distribution. The following statistical tests were utilized to compare the variables: chi-squared test for categorical variables; Student's
t test, or Mann-Whitney test in cases of continuous variables, either with normal distribution or not, respectively. The adopted
p value was 0.05.
RESULTS
In the already mentioned period, 241 children underwent liver transplant. Mean age of the children at the moment of transplantation was 36.9 ± 47.5 months, ranging from 4.5 to 215.2 months. Most transplant patients were female (
n = 133; 55.2%) and had a living donor (
n = 215; 89.2%). Biliary atresia was the disease that most frequently led to liver transplant (
n = 162; 67.2%), and tacrolimus was the most utilized immunosuppressive agent (
n = 203; 84.2%).
In a mean follow-up period of 41.4 ± 26.4 months, ranging from 0 to 125 months, 16 children (6.6%) developed lymphoma. The mean age of such children at the moment of diagnosis was 61.1 ± 32.0 months, ranging from 12.1 to 147.0 months, and most of them were male (
n = 9; 56.3%). The mean age at the moment of the transplant for children who developed lymphoma was lower than for children who did not (23.9 ± 18.9
versus 38.0 ± 48.9 months;
p = 0.02). No statistically significant difference was observed in the incidence of lymphomas in terms of sex, donor type, liver's disease leading to transplant or type of immunosuppressant treatment.
The time elapsed between liver transplant and diagnosis of lymphoma ranged from 6.8 to 103.5 months, corresponding to a mean of 37.2 ± 26.9 months. Only three children (18.8%) had the diagnosis in the first year after the transplantation (Figure 1).
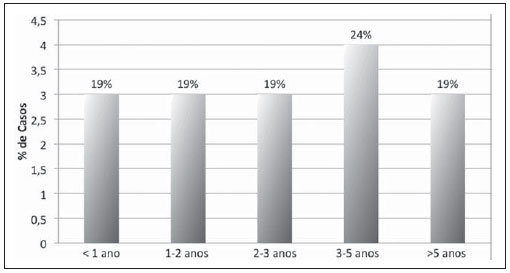
Figure 1. Distribution of cases of lymphoma in children submitted to liver transplantation according to time elapsed between transplant and diagnosis of lymphoma.
The most frequent symptoms were the following: abdominal pain, abdominal volume increase and cervical lymph node enlargement, present in four cases each, followed by prolonged fever in three cases. Two patients were asymptomatic and were diagnosed during routine follow-up.
The abdomen was most frequent tumor site (
n = 13; 81.3%), followed by chest and head and neck (
n = 4; 25.0% each). Abdominal imaging findings included masses and mesenteric and/or retroperitoneal lymphadenopathy in seven cases (Figure 2), intestinal wall thickening in three (Figure 3), focal hepatic lesions in two, and renal mass in one case (Figure 4). Chest involvement was characterized by the presence of mass or mediastinal lymphadenopathy in three cases and pulmonary mass in one case. All four cases of head and neck involvement were characterized by the presence of cervical lymphadenopathy.
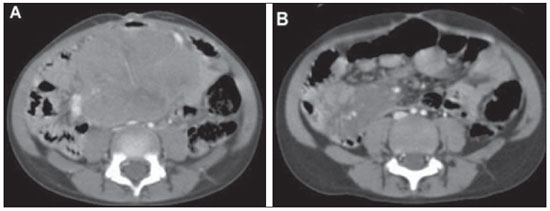
Figure 2. A six-year-old girl presenting heterogeneous abdominal mass involving mesenteric vessels, with histological diagnosis of non-Hodgkin's lymphoma. A: Pre-treatment contrast-enhanced CT image. B: Post-treatment contrast-enhanced CT image demonstrating decrease in the lesion dimensions.
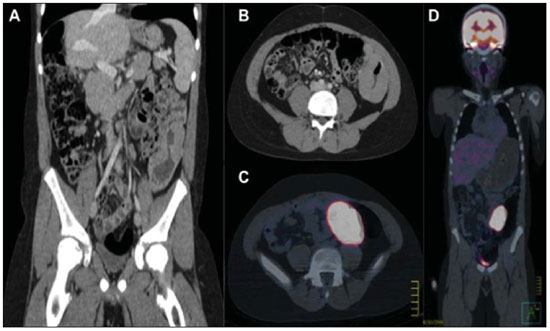
Figure 3. A 12-year-old boy presenting left colon wall thickening, with increased FDG uptake at PET-CT, whose histological diagnosis was non-Hodgkin's lymphoma. A: Coronal, contrast-enhanced CT section. B: Axial, contrast-enhanced CT section. C: Axial PET-CT section. D: Coronal PET-CT section.
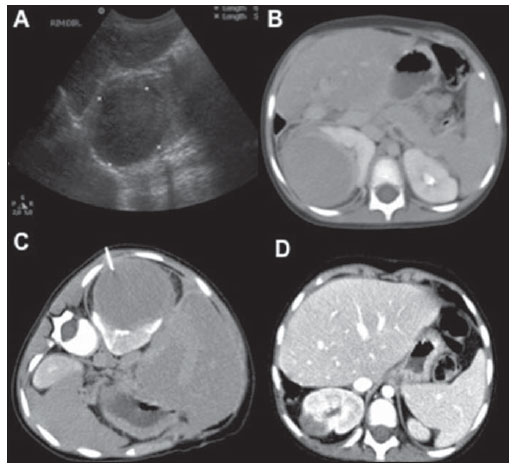
Figure 4. A three-year-old boy presenting a well-defined mass in the upper pole of his right kidney with histological diagnosis of non-Hodgkin's lymphoma. A: Ultrasonography. B: Contrast-enhanced CT image. C: CT-guided biopsy. D: Follow-up contrast-enhanced CT three months after treatment, demonstrating decrease in the lesion dimensions.
Non-Hodgkin lymphoma was the most common histological tumor type (
n = 14; 86.7%), with seven cases of Burkitt or Burkitt-like lymphoma, five cases of diffuse large B-cell lymphoma and one non-specific lymphoma. Hodgkin's lymphoma was the histological type in the other two cases (13.3%).
Until the end of data collection, three children (18.8%) remained under treatment, nine (56.3%) had been cured with treatment, and four (25.0%) died because of complications of lymphoma.
DISCUSSION
Studies in patients submitted to hepatic transplantation have demonstrated that lymphoma is the most common malignant neoplasm in this population, and that there is an inverse relationship between the incidence of PTLD and age at the moment of the liver transplantation(9,10). In the literature, the incidence of PTLD in pediatric patients submitted to liver transplant ranges from 4% to 15%(11,12).
PTLD may develop at any moment after the transplant but, according to reports in the literature, its incidence is highest within the first 12 months following transplant(13). In the present study, the mean was 37 months, with a maximum time of eight years and seven months. In the study developed by Fernandez et al., who have also evaluated children submitted to liver transplant, the mean time elapsed between the transplant and development of the disease was 25 months, ranging from seven to 47 months(11).
Age at the moment of the transplantation was the sole risk factor for development of lymphoma observed in the present study, which may be associated with previous contact of the patients with the Epstein-Barr virus (EBV). It is thought that the PTLD etiology is related to EBV-induced B-cell proliferation facilitated by the pharmacological immunosuppression of the host. Younger children present a lower rate of EBV positivity and for this reason they are more susceptible to the development of new infections after transplantation, which is associated with a high risk for development of PTLD(14). Because of the retrospective nature of the present study, the authors could not evaluate the serological status for EBV in all the patients before and after transplantation.
The clinical and radiological presentations of the disease are variable(15). In the early phases, most patients are asymptomatic, and later in the course of the disease, the symptoms are non-specific, so based only on clinical data it is difficult to establish the differential diagnoses with opportunistic infections and allograft rejection(4).
The anatomic distribution of PTLD is influenced by the allograph itself. Some authors have emphasized the post-transplant lymphoma propensity to occur in the same anatomic region of the transplanted organ, including involvement of the allograft itself(13,16). In the literature, abdominal involvement occurs in 50% to 75% of PTLD patients after kidney, liver or heart transplants(4). In the present study, the abdomen was also the most common site of involvement, followed by the chest and cervical region. The involvement of the central nervous system has already been described in the literature, but no case of such involvement was observed in the present study.
Imaging findings of lymphoproliferative disorders include lymphadenopathy, focal masses and diffuse organ infiltration(16,17). The most significant difference in terms of radiological manifestations of lymphoma occurring in the general population and in transplant patients is the relatively higher frequency of extranodal disease in the latter group. Extranodal sites of lymphomas in the abdomen include liver, small bowel, kidneys, mesenterium, adrenal glands, abdominal walls, colon and bladder(18). In the chest, pulmonary nodules or masses and mediastinal lymphadenopathy constitute the most common manifestations. In head and neck, the involvement is most frequently characterized by the presence of a focal mass in the Waldeyer's tonsillar ring and cervical lymphadenopathy(4).
Considering that PTLD frequently occurs in extranodal sites, PET-CT may be extremely useful in such cases(4). PET-CT performance is comparable to axial methods in the early staging of PTLD and demonstrates a higher potential to an earlier evaluation of treatment response than conventional methods(19). In the last years, whole body diffusion-weighted MRI has emerged as a relevant tool, providing functional information which may be utilized in the detection and characterization of malignant tumors. Such method presents the advantage of being a technique free of ionizing radiation that, even at low doses may increase the risk for secondary neoplasias in children, and has already demonstrated good results in the evaluation of lymphomas in pediatric patients(20).
Despite the appropriate treatment effectiveness and good response in most of cases, the disease can lead to death unless it is precociously diagnosed and treated. In the present study, the mortality rate reached 25%. Fernandez et al. have found a mortality rate of 18% in a similar population(11).
Finally, lymphomas are relatively uncommon and potentially fatal complications that may occur at any moment after liver transplant in children and that present variable clinical and imaging findings. Radiologists must know the manifestation of this disease to allow an early diagnosis, which might change the management and clinical outcome of the disease. The main objectives of imaging methods include the detection of the disease, evaluation of its extent, guidance in percutaneous biopsy and evaluation of the treatment response.
REFERENCES
1. Zafar SY, Howell DN, Gockerman JP. Malignancy after solid organ transplantation: an overview. Oncologist. 2008;13:769—78.
2. Taylor AL, Marcus R, Bradley JA. Post-transplant lymphoproliferative disorders (PTLD) after solid organ transplantation. Crit Rev Oncol Hematol. 2005;56:155—67.
3. Pickhardt PJ, Siegel MJ, Hayashi RJ, et al. Posttransplantation lymphoproliferative disorder in children: clinical, histopathologic, and imaging features. Radiology. 2000;217:16—25.
4. Borhani AA, Hosseinzadeh K, Almusa O, et al. Imaging of posttransplantation lymphoproliferative disorder after solid organ transplantation. Radiographics. 2009;29:981—1002.
5. Harris NL, Swerdlow SH, Frizzera G, et al. Post-transplant lymphoproliferative disorders. In: Jaffe ES, Harris NL, Stein H, et al., editors. World Health Organization Classification of Tumours: pathology and genetics of tumours of haematopoietic and lymphoid tissues. Lyon: IARC; 2001. p. 264—9.
6. Scarsbrook AF, Warakaulle DR, Dattani M, et al. Post-transplantation lymphoproliferative disorder: the spectrum of imaging appearances. Clin Radiol. 2005;60:47—55.
7. Burney K, Bradley M, Buckley A, et al. Posttransplant lymphoproliferative disorder: a pictorial review. Australas Radiol. 2006;50:412—8.
8. Dhillon MS, Rai JK, Gunson BK, et al. Post-transplant lymphoproliferative disease in liver transplantation. Br J Radiol. 2007;80:337—46.
9. Aberg F, Pukkala E, Höckerstedt K, et al. Risk of malignant neoplasms after liver transplantation: a population-based study. Liver Transplant. 2008;14:1428—36.
10. Jain A, Nalesnik M, Reyes J, et al. Posttransplant lymphoproliferative disorders in liver transplantation: a 20-year experience. Ann Surg. 2002;236:429—37.
11. Fernández MC, Bes D, De Dávila M, et al. Post-transplant lymphoproliferative disorder after pediatric liver transplantation: characteristics and outcome. Pediatr Transplant. 2009;13:307—10.
12. Jeon TY, Kim JH, Eo H, et al. Posttransplantation lymphoproliferative disorder in children: manifestations in hematopoietic cell recipients in comparison with liver recipients. Radiology. 2010;257:490—7.
13. Opelz G, Döhler B. Lymphomas after solid organ transplantation: a collaborative transplant study report. Am J Transplant. 2004;4:222—30.
14. Wu JF, Ho MC, Ni YH, et al. Timing of Epstein-Barr virus acquisition and the course of posttransplantation lymphoproliferative disorder in children. Transplantation. 2009;87:758—62.
15. Caldas FAA, Motomiya CT, Silva HC. Análise de achados de imagem e alterações clínicas em pacientes com linfoma. Radiol Bras. 2002;35:71—5.
16. Donnelly LF, Frush DP, Marshall KW, et al. Lymphoproliferative disorders: CT findings in immunocompromised children. AJR Am J Roentgenol. 1998;171:725—31.
17. Borba AMV, Monteiro AMV, Lima CMAO, et al. Aspectos da tomografia computadorizada no linfoma em pacientes abaixo de 20 anos de idade. Radiol Bras. 2007;40:87—92.
18. Pickhardt PJ, Siegel MJ. Posttransplantation lymphoproliferative disorder of the abdomen: CT evaluation in 51 patients. Radiology. 1999;213:73—8.
19. von Falck C, Maecker B, Schirg E, et al. Post transplant lymphoproliferative disease in pediatric solid organ transplant patients: a possible role for [18F]-FDG-PET(/CT) in initial staging and therapy monitoring. Eur J Radiol. 2007;63:427—35.
20. Nava D, Oliveira HCO, Luisi FA, et al. Aplicação da ressonância magnética de corpo inteiro para o estadiamento e acompanhamento de pacientes com linfoma de Hodgkin na faixa etária infanto-juvenil: comparação entre diferentes sequências. Radiol Bras. 2011;44:29—34.
1. MDs, Residents, Hospital A. C. Camargo, São Paulo, SP, Brazil.
2. MD, Master, Hospital A. C. Camargo, São Paulo, SP, Brazil.
3. MD, PhD, Director, Department of Imaging Diagnosis, Hospital A. C. Camargo, São Paulo, SP, Brazil.
Mailing Address:
Dr. Almir Galvão Vieira Bitencourt
Hospital A. C. Camargo, Departamento de Imagem
Rua Professor Antônio Prudente, 211, Liberdade
São Paulo, SP, Brazil, 01509-010
E-mail: almirgvb@yahoo.com.br
Received December 4, 2011.
Accepted after revision February 6, 2012.
Study developed at Department of Imaging, Hospital A. C. Camargo, São Paulo, SP, Brazil.
 Vol. 45 nº 1 - Jan. /Feb. of 2012
Vol. 45 nº 1 - Jan. /Feb. of 2012



