INTRODUCTION
Cancerization of lobules by ductal carcinoma in situ (DCIS) is a pathological pattern that can be defined as the farthest extension of DCIS into the lobules and interlobular ducts(1). The identification of cancerization of the lobules is important as it is considered a significant, independent predictor of invasion in patients with biopsy- diagnosed DCIS(2).
To our knowledge, no previous studies have assessed the mammographic appearance of lobular cancerization by DCIS. We aimed to describe the mammographic appearance of cancerization of lobules by DCIS, by correlating imaging and histological findings.
MATERIALS AND METHODS
This retrospective study was based on a review of histopathological reports of 135 patients who underwent breast biopsy in two hospitals, in the period between 1998 and 2006, and who received a final diagnosis of DCIS. The diagnosis of cancerization of lobules by DCIS was confirmed by histopathology in 12 of the 135 patients. Two cases were excluded because histopathological sections were not available to correlate pathological and mammographic findings. The institutional review board of our hospital approved the study.
The mammograms (craniocaudal and mediolateral views) were obtained with different high-resolution film-screen systems (GE 600T and GE DMR; GE Medical Systems, Milwaukee, WI, USA) in the two hospitals. Additional views, including magnification techniques, were performed at the discretion of the mammographer. In all the cases, specimen radiographs were obtained to confirm the excision of microcalcifications, and to guide the histological examination. All the mammograms were retrospectively reviewed and categorized by two experienced breast radiologists, with no previous knowledge of the histological findings. At mammography, microcalcifications were categorized in terms of morphology, distribution, and number, and were correlated with the histopathological pattern. Calcification shapes were classified as: 1) round – including punctate, round or oval calcifications besides those which are very sharply defined; 2) amorphous calcifications, which are indistinct, flake-shaped, or hazy in appearance; 3) pleomorphic or heterogeneous calcifications, irregularly shaped, varying in size and shape, and less than 0.5 mm in diameter; 4) fine, linear, branching calcifications, including thin, irregular calcifications (< 1 mm)(3,4). According to distribution pattern, the calcifications were classified as: 1) ductal – linearly distributed calcifications with a triangular/trapezoidal shape; 2) lobular – clusters of round calcifications; 3) indefinite – this group defines a distribution pattern that cannot be classified as lobular or ductal(3,5). Calcification clusters were classified as follows: up to 10 calcifications; from 10 to 20; and more than 20 calcifications.
Histological specimens derived from a combination of core biopsies and surgical excisions were stained with hematoxylin and eosin for pathological evaluation and staging. Pathology specimens were reviewed by an experienced breast pathologist with no previous knowledge of the mammographic appearances, who classified the specimens according to their predominant histological subtype. The histopathological classification of DCIS was based on the descriptions of Tavassoli(6) and Rosen(7), and classified as micropapillary, cribriform, comedo, solid, and papillary. Lobular cancerization was defined as the partial involvement by DCIS cells of a group of lobules within a terminal duct lobular unit, so that such lobules be recognizable as part of a single unit(6,8).
RESULTS
Nine cases (90%) presented clusters of round microcalcifications, and one (10%) had round and linear calcifications. The distribution of calcifications was defined as lobular in all of the cases.
In the nine cases with clusters of round microcalcifications the histopathology showed: four cases of cribriform DCIS (Figure 1), two cases of comedo DCIS (Figure 2), one case of solid DCIS, one case of cribriform associated with solid DCIS (Figure 3), and one case of cribriform associated with solid and comedo DCIS. The one case with round and linear calcifications had a histological classification of cribriform DCIS. With respect to the number of microcalcifications, nine cases presented more than 20, and only one case showed less than 10 microcalcifications.
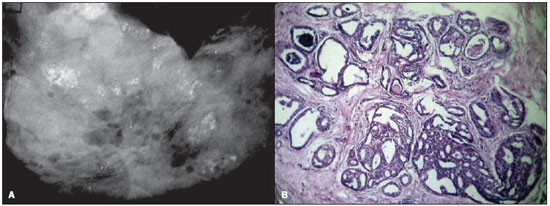
Figure 1. A: Mammography of a 41-year-old woman with ductal carcinoma in situ. The image shows multiple clusters of round microcalcifications with a lobular pattern of distribution. B: High-power photomicrograph (original magnification, x40; hematoxylin-eosin stain) demonstrates a lobular structure with atypical cells, and enlarged acinus, presenting secretions and calcifications. The histopathological findings were consistent with cancerization of the lobule by cribriform DCIS.
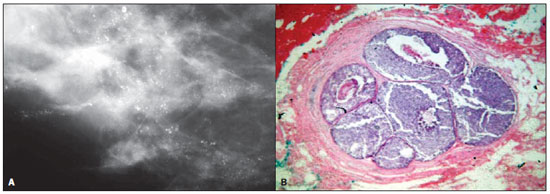
Figure 2. A: Mammography of a 57-year-old woman with ductal carcinoma in situ. The image shows clusters of punctuate microcalcifications which vary in size, forming bunches of calcifications. B: High-power photomicrograph (original magnification, x40; hematoxylin-eosin stain) demonstrates a well defined, lobular structure with enlarged ducts, atypical cells, and central necrosis, consistent with cancerization of lobule by comedo DCIS.
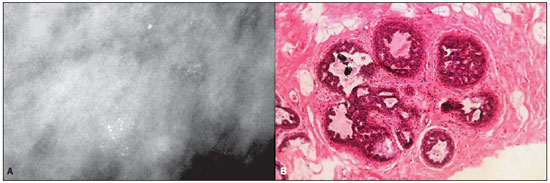
Figure 3. A: Mammography of a 38-year-old woman with ductal carcinoma in situ. The image demonstrates clusters of round, mostly fine, granular
microcalcifications, with a lobular morphology. B: High-power photomicrograph (original magnification, x40; hematoxylin-eosin stain) demonstrates a lobular structure, with atypical cells with a cribriform pattern, presenting calcifications within enlarged acinus. The histopathological findings were consistent with cancerization of lobule by cribriform and solid DCIS.
Cancerization of lobules is a histopathological pattern described in cases where a DCIS partially involves the terminal duct lobular units, which progressively become enlarged and distended. Continued expansion of malignant cells into the lobules results in destruction of the normal architecture, and finally ending in duct-like structures of DCIS(1,8). Cancerization of lobules is regarded as an important predictor of invasion and may be associated with an increased risk of residual DCIS in cases of re-excision and recurrence(2). Moreover, the recognition of possible mammographic patterns indicating the presence of DCIS with cancerization of lobules may help to improve the differential diagnosis of breast microcalcifications.
Breast microcalcifications usually follow the architecture of the site where they are formed, since linearly distributed calcifications of DCIS are formed within the ducts(3,4,8). However, DCIS is also known to extend into the lobules leading to their cancerization. Calcifications of DCIS are typically fine, linear concretions that branch with the duct(4); however, in this study, all the mammograms showed clusters of microcalcifications in a lobular distribution. The morphology of such microcalcifications was a result of calcifications formed in the remnants of ‘burned-out’ lobules destroyed by their cancerization. Based on such results, we hypothesize that, with the invasion of the lobules by DCIS, the shape of the calcifications follow the architecture of the lobular acini, forming clusters of round calcifications. Usually, clusters of small, round, or oval calcifications distributed in a lobular pattern are associated with benign processes, such as adenosis, peripheral duct papillomas, and other benign breast conditions. However, our results demonstrate that this pattern may also be associated with DCIS with lobular cancerization.
Huo et al.(2) have evaluated 200 consecutive patients with core-needle biopsydiagnosed DCIS to determine the predictors of tumor invasion. Such authors have observed that the most significant predictors of invasion in patients with DCIS are the presence of a mass lesion, a lesion greater than 1.5 cm in diameter, and the presence of lobular cancerization. In addition, they have concluded that each of such variables, except nuclear grade, is an independent predictor of invasion. Corroborating these conclusions, Renshaw(10) has reported that patients with DCIS measuring > 4 mm in the largest dimension, together with lobular extension, had an increased risk for invasion on final excision.
The present study has some limitations. It is a non-randomized, retrospective analysis of a small cohort of patients. Additionally, the cases of cancerization of lobules were selected by means of histopathological analyses, and our study population included only those patients whose histopathological diagnosis was confirmed. In spite of such limitations, this is the first study assessing the mammographic appearance of lobular cancerization by DCIS.
In all of the cases of our cohort, the mammographic evaluation of patients with DCIS presenting cancerization of lobules showed clusters of microcalcifications in a lobular distribution. Although clusters of round calcifications are typically associated with a benign process, cancerization of lobules by DCIS may produce a similar pattern, thus mimicking a benign condition.
REFERENCES
1. Azzopardi JG. Underdiagnosis of malignancy. In: Bennington JL, editor. Problems in breast pathology. London: WB Saunders; 1979. p. 192–239.
2. Huo L, Sneige N, Hunt KK, et al. Predictors of invasion in patients with core-needle biopsy-diagnosed ductal carcinoma in situ and recommendations for a selective approach to sentinel lymph node biopsy in ductal carcinoma in situ. Cancer. 2006;107:1760–8.
3. Lanyi M. Diagnosis and differential diagnosis of breast calcifications. Berlin: Springer-Verlag; 1986.
4. Kopans DB. Breast imaging. Philadelphia: Lippincott Williams & Wilkins; 2007.
5. Lanyi M, Neufang KF. Possibilities and limitations of the differential diagnosis of grouped intramammary microcalcifications. Rofo. 1984:141:430–8.
6. Tavassoli FA. Ductal intraepithelial neoplasia: risk factors for subsequent development of invasive carcinoma. In: Tavassoli FA, editor. Pathology of the breast. Stanford: Appleton & Lange; 1999. p.205–312.
7. Rosen PP. Rosen’s breast pathology. New York: Lippincott-Raven; 1978.
8. Vicini FA, Goldstein NS, Kestin LL. Pathologic and technical considerations in the treatment of ductal carcinoma in situ of the breast with lumpectomy and radiation therapy. Ann Oncol. 19910:883–90.
9. Renshaw AA. Predicting invasion in the excision specimen from breast core needle biopsy specimens with only ductal carcinoma in situ. Arch Pathol Lab Med. 2002;126:39–41.
1. Associate Professor, Department of Radiology, Universidade Federal Fluminense (UFF), Niterói, RJ, Brazil.
2. Fellow PhD degree in Radiology, Universidade Federal do Rio de Janeiro (UFRJ), Rio de Janeiro, RJ, Brazil.
3. MD, Radiologist, Department of Radiology, Universidade Federal Fluminense (UFF), Niterói, RJ, Brazil.
4. MD, Pathologist, Department of Pathology, Universidade Federal Fluminense (UFF), Niterói, RJ, Brazil (In memoriam).
5. Full Professor, Department of Radiology, Universidade Federal Fluminense (UFF), Niterói, RJ, Adjunct Coordinator, Course of Post-Graduation in Radiology, Universidade Federal do Rio de Janeiro (UFRJ), Rio de Janeiro, RJ, Brazil.
Mailing Address:
Dra. Taísa Davaus Gasparetto
Rua Lopes Trovão, 88, ap. 1702-B, Icaraí
Niterói, RJ, 24220-071, Brazil
E-mail: taisadavaus@gmail.com
Received February 23, 2011.
Accepted after revision May 14, 2011.
Study developed at Universidade Federal Fluminense (UFF), Niterói, RJ, and Universidade Federal do Rio de Janeiro (UFRJ), Rio de Janeiro, RJ, Brazil.
 Vol. 44 nº 5 - Sep. / Oct. of 2011
Vol. 44 nº 5 - Sep. / Oct. of 2011


