A male, 16-year-old, asymptomatic patient presented murmur at cardiac auscultation. Echocardiography demonstrated that such a murmur was related to morphofunctional alteration in the valve apparatus of the right heart. The patient was referred to undergo cardiac magnetic resonance imaging (CMRI).
Images description
Figure 1. Images acquisition with ECGgating, in cine-Fiesta sequence (SSFP), long axis four-chambers. (
A) at diastole, (
B) systole and (
C) mid-diastole. Note the apical displacement of the tricuspid valve apparatus into the right ventricle, with increase in volume of the right atrium and partial fusion of the leaflets with the septal heart muscle, causing deformity of the left ventricle apex.
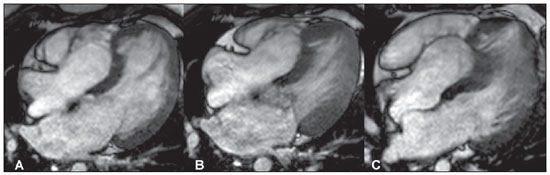
Figure 1. Images acquisition with ECG-gating, in cine-Fiesta sequence (SSFP), long axis four-chambers. (A) at diastole, (B) systole e (C) mid-diastole.
Images acquisition with ECGgating. Delayed enhancement, long axis four-chambers. (
A) and (
B), different planes. Note the positive delayed enhancement on the middle third of the septum.
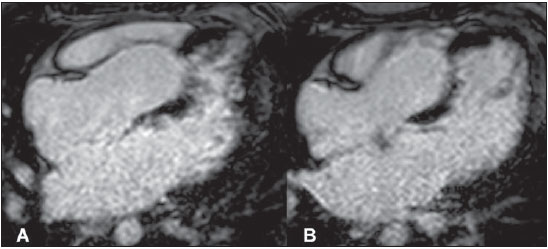
Figure 2. Images acquisition with ECG-gating. Delayed enhancement, long axis four-chambers. (A) and (B), different planes.
Ebstein’s anomaly with septal fibrosis
COMMENTS
For more than 20 years CMRI has been utilized as a reference method in the study of congenital cardiopathies, particularly those of the right ventricle. The capability to perform a functional evaluation with measurements of flow and gradient in association with an anatomical study of the heart and great vessels allows an almost complete evaluation for the purposes of surgical planning and patients followup(1,2).
Ebstein’s anomaly is a rare congenital cardiopathy found in only 0.5–1% of cases in necropsies and may cause cyanosis at birth. Such anomaly is characterized by a tricuspid valve malformation associated with its displacement towards the apex of the right ventricle (atrialization)(1,2).
The clinical condition progression varies and depends upon the degree of valve dysfunction and malformation. Generally, death in the neonatal period is most commonly caused by severe heart failure. After the neonatal period, arrhythmia and fatigue are the most frequent findings. Arrhythmias tend to present a higher incidence because of abnormalities in the cardiac conduction system, with a certain frequency generating cases of Wolff-Parkinson-White syndrome, premature atrial or ventricular contractions, or presence of flutter/atrial fibrilation. Despite the pattern of involvement of the right ventricle, the left side may also be affected, particularly because of abnormalities in the mitral valve and in the left ventricular contractility(2,3).
The possibility of coexistence with other congenital cardiopathies that may affect the cardiac skeleton or the functional mechanics of the heart, such as interatrial communication, pulmonary valve stenosis, pulmonary atresia or non-compaction of the myocardium, among others, makes cardiac MRI a noninvasive diagnostic method to be performed before any other therapeutic procedure(1,4,5).
Volumetric study of heart’s chambers should be performed by means of a reproducible method, with low interobserver variability and without risks, particularly those originated by radiation exposure, since in many cases patients must undergo long-life follow-up(1).
The evaluation of myocardial delayed enhancement using CMRI allows the detection of fibrotic areas in the myocardial tissue. Recently, Nakamura et al.(5) reported the presence of myocardial fibrosis in the atrialized portion of the right ventricle in a 69-year-old patient. In the present case, the same finding was identified, but in a 16- year-old patient. However, the clinical implications related to the survival of patients with Ebstein’s anomaly with fibrosis in the childhood or youth are still to be understood.
In cases involving late diagnosis, the most characteristic imaging finding is the disproportion between right and left cavities (Figure 3).
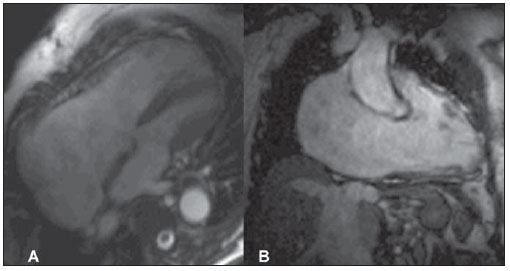
Figure 3. Images acquisition with ECG-gating, in cine-MRI sequence (SSFP), long-axis four-chambers (A) and long-axis two-chambers of left ventricle (B). Note the great dilatation of the right atrium and ventricle. Study performed at Hospital Pro-Cardíaco, Rio de Janeiro, RJ, Brazil.
There are different modalities of treatment for Ebstein’s anomaly. In the neonatal period, as the anomaly is very severe and the patient remains cyanotic even under aggressive drug and ventilatory therapy, transplant is the best choice. However, transplants have been poorly successful because of the reduced size of the lungs. After the neonatal period, treatment options increase. For many years, the treatment of choice was only tricuspid valve replacement in association with reduction of the atrialized portion of the left ventricle. Recently, cone reconstruction has been more frequently used. Such procedure involves the creation of a tunnel-type structure with the tricuspid valve to help minimize postprocedural regurgitation. Interatrial septal defect, if present, is closed and the size of the right atrium is reduced. During the surgical procedure, it is necessary to reduce the number of probable pathological circuits of electrical conduction(2,6–12).
Final considerations
Surgical treatment has been proposed in innumerable cases(6,7,9–11), but clinical evidences associated with cardiac MRI in the preoperative stratification will facilitate the understanding of the clinical outcomes for patients with Ebstein’s anomaly.
Cardiac MRI is essential in the noninvasive preoperative diagnosis of this condition. It is the authors’ opinion that the utilization of such imaging modality can influence the survival, particularly in the case of patients who will be submitted to electrophysiological interventions.
REFERENCES
1. Yalonetsky S, Tobler D, Greutmann M, et al. Cardiac magnetic resonance imaging and the assessment of ebstein anomaly in adults. Am J Cardiol. 2011;107:767–73.
2. Link KM, Herrera MA, D’Souza VJ, et al. MR imaging of Ebstein anomaly: results in four cases. AJR Am J Roentgenol. 1988;150:363–7.
3. Beerepoot JP, Woodard PK. Case 71: Ebstein anomaly. Radiology. 2004;231:747–51.
4. Bagur RH, Lederlin M, Montaudon M, et al. Images in cardiovascular medicine. Ebstein anomaly associated with left ventricular noncompaction. Circulation. 2008;118:e662–4.
5. Nakamura I, Kotooka N, Komori Y, et al. Ebstein anomaly by cardiac magnetic resonance imaging. J Am Coll Cardiol. 2009;53:1568.
6. Brown ML, Dearani JA, Danielson GK, et al. Functional status after operation for Ebstein anomaly: the Mayo Clinic experience. J Am Coll Cardiol. 2008;52:460–6.
7. Brown ML, Dearani JA, Danielson GK, et al. The outcomes of operations for 539 patients with Ebstein anomaly. J Thorac Cardiovasc Surg. 2008;135:1120–36,36 e1–7.
8. Nakata T, Fujimoto Y, Hirose K, et al. Norwood procedure combined with the Starnes procedure. Ann Thorac Surg. 2008;86:1378–80.
9. Van Arsdell G. Can we modify late functional outcome in Ebstein anomaly by altering surgical strategy? J Am Coll Cardiol. 2008;52:467–9.
10. Brown ML, Dearani JA, Danielson GK, et al. Comparison of the outcome of porcine bioprosthetic versus mechanical prosthetic replacement of the tricuspid valve in the Ebstein anomaly. Am J Cardiol. 2009;103:555–61.
11. Boston US, Goldberg SP, Ward KE, et al. Complete repair of Ebstein anomaly in neonates and young infants: a 16-year follow-up. J Thorac Cardiovasc Surg. 2011;141:1163–9.
12. Liu J, Qiu L, Zhu Z, et al. Cone reconstruction of the tricuspid valve in Ebstein anomaly with or without one and a half ventricle repair. J Thorac Cardiovasc Surg. 2011;141:1178–83.
1. PhD, Professor, Universidade Federal Fluminense (UFF), Niterói, RJ, Advisor in Cardiac Imaging, Plani Diagnósticos Médicos por Imagem, São José dos Campos, SP, Brazil.
2. MD, Radiologist, Coordinator for the Group of Internal Medicine, Plani Diagnósticos Médicos por Imagem, São José dos Campos, SP, Brazil.
3. Medical Director, Plani Diagnósticos Médicos por Imagem, São José dos Campos, SP, Brazil.
Mailing Address:
Dr. Marcelo Souto Nacif
4853 Cordell Avenue, 419
Bethesda, MD, USA
E-mail: msnacif@yahoo.com.br
Study developed at Plani Diagnósticos Médicos por Imagem, São José dos Campos, SP, Brazil.
 Vol. 44 nº 5 - Sep. / Oct. of 2011
Vol. 44 nº 5 - Sep. / Oct. of 2011


