Radiologia Brasileira - Publicação Científica Oficial do Colégio Brasileiro de Radiologia
AMB - Associação Médica Brasileira CNA - Comissão Nacional de Acreditação
 Vol. 44 nº 3 - May / June of 2011
Vol. 44 nº 3 - May / June of 2011
|
ORIGINAL ARTICLE
|
|
Repercussions of previous cesarean uterine scar at uterine arteries Doppler velocimetry between the 26th and 32nd gestational weeks |
|
|
Autho(rs): Octávio de Oliveira Santos Filho1; Luciano Marcondes Machado Nardozza2; Edward Araujo Júnior3; Luiz Camano4; Antonio Fernandes Moron4 |
|
|
Keywords: Pregnancy; Uterine scar; Previous cesarean section; Uterine arteries Doppler velocimetry. |
|
|
Abstract: INTRODUCTION
The adverse effects that a previous cesarean uterine scar may cause highlight the increasing frequency of placenta previa, placenta accreta, ectopic pregnancy at the scar niche and uterine rupture, in future pregnancies(1—5). Anatomic changes result from decreased local vascularization caused by fibrosis, which is greater in elective cesarean sections performed without appropriate formation of the lower uterine segment(6). Several imaging methods have been utilized in the assessment of the integrity of this region in uteri with scars from previous cesarean section. In the seventies, hysterography played a relevant role in demonstrating defective scarring (presence of scar niche), recently replaced by sonohysterography, hysteroscopy and magnetic resonance imaging, also performed out of the gestational period(7—9). Ultrasonography, a safe and noninvasive method(10), has been utilized during the gestational period with this purpose since the eighties(2,11,12). It is possible that previously performed cesarean sections, whether electively or during labor, cause differences in the evaluation of the uteroplacental flow. The choice of the uterine artery for dopplervelocimetric evaluation of the uteroplacental circulation offers better accuracy than small arteries such as the arcuate or radial arteries, not only for being of easier insonation, but also because it reflects the total distal resistance of the vascular flow of this circulation(13). During pregnancy, the uterine artery flow is 10 times greater than the non-pregnant flow, thus enhancing the blood supply to the fetal-placental unit. Such increase in flow depends on the trophoblastic invasion of spiral arteries which occurs in phases, starting at the decidual segments and later at the myometrial segments(14,15). The irregularity in the physiological process of the trophoblast invasion is associated with placental insufficiency, occurrence of pre-eclampsia, placental abruption, fetal growth restriction and/or fetal death(16—18). Relevant factors such as placental location must be considered during the performance of exams. Additionally, other important factors are parity and presence of uterine scars, particularly those caused by previous cesarean section, which could change the local circulation to an extent capable of affecting the results from uterine arteries dopplervelocimetry. The present study is aimed at assessing in current pregnancies the possible repercussions of previous cesarean section scars on uterine arteries dopplervelocimetry between the 26th and 32nd gestational weeks, considering the characteristics of the indication for cesarean section: electively or during labor. MATERIALS AND METHODS The present prospective, cross sectional study was developed between April, 2007 and November, 2009, with pregnant women with one previous cesarean section selected at the first prenatal visit at the Hospital Celso Pierro — Pontifícia Universidade Católica de Campinas (PUC-Campinas), SP, Brazil. All the patients who agreed to participate in the study duly signed a term of free and informed consent. The study was approved by the Committee for Ethics in Research of Universidade Federal de São Paulo (Unifesp). The patients with a single previous parturition, were divided into three groups: GA — pregnant women with a previous scar resulting from elective cesarean section; GB — pregnant women with a previous scar resulting from cesarean section performed during labor; GC — pregnant women whose single previous delivery was vaginal, constituting the control group. The gestational age was determined by the last menstrual period (LMP), reported with certainty by the patient at the moment of the examination and confirmed by first trimester ultrasonography through the crown-rump length (CRL). For the purpose of division of the patients into the three groups, they were asked whether the previous cesarean section was performed before or after the onset of uterine contractions. The homogeneity of the groups was measured by age, ethnicity and body mass index (BMI). The considered age (in years) was that reported by the patient on the day of the US scan. The BMI calculation was based on the women habitual pre-pregnancy weight and height. As regards ethnicity the women were classified as white, mulatto, black or other. Based on the findings by Guidoni et al.(19) the previous suture type was not taken into consideration in the present study. Only primipara pregnant women aged between 18 and 35 years were included in the present study. Exclusion criteria were the following: chronic, gestational hypertension or pre-eclampsia; fetal growth restriction; antiphospholipid antibody syndrome and collagenoses; vaginal bleeding in the current pregnancy; low lying placenta; alcoholic patients, smokers or illicit drug users; patients with BMI > 35 at the beginning of pregnancy; multiple gestation; pregnancy resulting from assisted fertilization; endometriosis; leiomyoma or other gynecopathy that might potentially affect the uterine flow; history of repetitive miscarriages; endometritis in previous pregnancy; polyhydramnios; patients with more than one previous cesarean section; previous cesarean section in preterm pregnancy; previous longitudinal cesarean section and primipaternity. A Sonoace 8000 Live (Medison; Seul, Korea) apparatus with a 3.5 MHz sector transducer was utilized for the dopplervelocimetry study of the uterine arteries by abdominal approach, between the 26th and 32nd gestational weeks. The scans were performed with the patients in the semi-Fowler position and the transducer was placed on the longitudinal plane along the inguinal region, at 2 to 3 cm from the anterior superior iliac spine, directed to the lateral wall of the uterus, at the level of the internal uterine cervical os. The uterine arteries were identified at color Doppler and insonated at approximately 1 cm from the crossing point with the external iliac artery(20,21). A 100 Hz filter was utilized and the waves quality was maximized by the use of the smallest insonation angle (60º). The analysis of the waveforms was only performed when at least three uniform and consecutive waveforms were obtained(13) (Figure 1). From the obtained and selected waveform, the pulsatility index (PI) was measured. The S point is the systolic velocity peak and D is the final diastolic velocity (Figure 2). The PI was automatically calculated by the system. The procedure was repeated three times for each uterine artery, in order to obtain the mean PI value for each side. Subsequently, the mean between the two obtained values was calculated, resulting in a final PI value for each pregnant woman, thus eliminating the interference of the placental location(16). 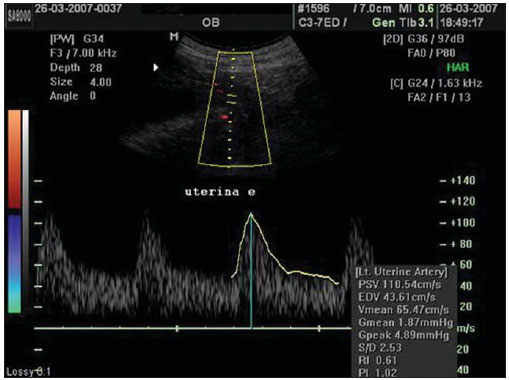 Figure 1. Uterine artery Doppler ultrasonography with uniform waveforms. 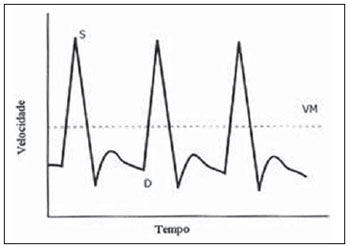 Figure 2. Diagram representing uterine artery Doppler ultrasonography representing the peak of systolic velocity and final diastolic velocity. The data were recorded on an Excel 2003 (Microsoft; Redmond, WA, USA) worksheet and were then analyzed by means of the statistical package SPSS version 13.0 for Windows (SPSS Inc.; Chicago, IL, USA). The main quantitative variable of interest, PI, was described by means of mean value, standard deviation (SD), median, minimum and maximum values, thus allowing the comparison of the values obtained for the different groups. The same statistical measurements were utilized to characterize the groups according to the following variables: age, BMI and respective gestational ages. As regards ethnicity, the groups were described by means of frequency distribution. The studied groups were compared by means of variance analysis (ANOVA) as the variable distribution presented a normal distribution (Gaussian), as in the cases of the variables of interest such as age, BMI and gestational ages. Whenever evidence of statistically significant difference was observed between the groups identified by ANOVA (p < 0.05), the Duncan method for multiple comparisons was utilized, with the purpose of recognizing the intergroup differences. As regards the mean PI values, the groups were compared by means of the Kruskal-Wallis test. A significance level of (p) < 0.05 was utilized. RESULTS The present study sample included 45 pregnant women, divided into three groups: 17 elective cesarean sections, 14 cesarean sections in labor and 14 in the control group. As regards the patients’ age, no statistically significant difference was observed between the three groups (p = 0.95). The mean age was around 26 years. Approximately 80% of the patients were white, in the three groups, with very similar ethnic profiles, with no statistically significant difference (p =1.00). The BMI also did not present evidences of statistically significant intergroup differences (p = 0.33), with a mean value close to 23. Gestational age at the time of ultrasonography examination did not present any evidence of statistically significant intergroup difference, with a mean gestational age of 27.9 weeks. As regards PI values, in group A it ranged from 0.60 to 1.60 (mean: 0.90; SD: 0.29); in the group B, from 0.53 to 1.43 (mean: 0.87; SD: 0.24); and in group C, from 0.65 to 1.65 (mean: 1.01; SD: 0.37), with no evidence of statistically significant intergroup differences (p = 0.6329) (Table 1). 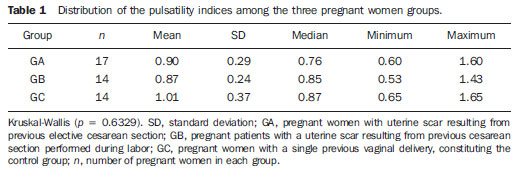 DISCUSSION The increase in the number of cesarean sections is noticeable in the entire world since the 1970’s. Brazil is currently one of the countries with the highest index of cesarean sections, with significant increase over the years, oscillating from 14.6% in the early 1970’s to 41.8% in 2005(22). With the increase in the index of cesarean sections, the evaluation of uterine scars, particularly those resulting from previous Cesarean section, gained great importance in obstetric assistance, as it can change the local anatomy and affect the obstetric outcomes, predisposing the patients to disorders such as placenta previa, placental accreta, scar dehiscence, uterine rupture or ectopic pregnancy at the scar niche(1,2,20,23). Zimmer et al.(24) have evaluated, by means of transvaginal US, the location of uterine scar resulting from previous cesarean section, between the 14th and 16th gestational weeks, to compare the uterine scar in patients whose previous cesarean section was performed electively or during labor. They have separated the patients by asking them whether the previous cesarean sections were performed before or after the onset of uterine contractions. They considered the sonographic image corresponding to the scar as a hypoechogenic line seen at the isthmocervical region. The results have demonstrated that such line was most easily visualized when the previous cesarean section was performed during labor (75.7% versus 52.7%; p = 0.001) and that the scar was most distant from the internal cervical os in the same conditions (17.9 versus 14.6 mm). The prematurity variable had an influence only in cases where the previous cesarean section was elective, and the scar was closer to the uterine body. Thus, the authors have concluded that when the cesarean section is performed during the labor, it is done at the level of the isthmus (cervical structure); on the other hand, when it is performed out of the labor section includes the myometrial structure. Such findings are in agreement with the physiological process of cervical effacement, shortening and softening during uterine contractions, when the cervix becomes part of the segment. However, such a process is still to be histologically defined. In this study, the parity or the number of previous cesarean sections have not been considered. The results from such study lead to important clinical reflections: elective cesarean sections should be avoided whenever possible in the appropriate assistance to delivery, thus decreasing the aggravation to the uterine matrix and decreasing the risk for placenta previa, placenta accreta, uterine rupture or ectopic pregnancy at the scar niche. Oosterhof et al.(13) have elected the uterine arteries as the best option to study the uteroplacental circulation, as such arteries provide better accuracy than the small arteries such as the arcuate, straight and radial arteries, not only for being easier to be insonated, but also for reflecting the total distal resistance of the vascular flow in this circulation. However, no study on the evaluation of the uterine scar vascularization by means of ultrasound Doppler was found in literature. In the present study, comparing the findings at uterine arteries dopplervelocimetry, no changes in the pulsatility indices of such arteries were found among the three studied groups. Based on such results, the authors belief that the uterine matrix circulation is of such a magnitude, that it cannot be changed by scarring process secondary to cesarean sections. Thus, the circulatory damages caused by collagen deposition would be restricted only to the scar location or to the microcirculation, impairing its evaluation by uterine arteries dopplervelocimetry. Possibly three-dimensional ultrasonography, for allowing the local circulation quantification, may be a valuable method to study circulatory changes of uterine scars. However new studies will be required to confirm such assumptions. CONCLUSIONS No repercussion of previous cesarean section scars was observed at dopplervelocimetry of uterine arteries performed in the period from the 26th to the 32nd gestational weeks. REFERENCES 1. Laughon SK, Wolfe HM, Visco AG. Prior cesarean and the risk for placenta previa on second-trimester ultrasonography. Obstet Gynecol. 2005;105(5Pt1):962—5. 2. Rezende Filho J. Avaliação do segmento inferior. Femina. 2006;34:791—2. 3. Ash A, Smith A, Maxwell D. Caesarean scar pregnancy. BJOG. 2007;114:253—63. 4. Corrêa MA, Orsatto Jr S, Torloni MR. Influência da cesárea anterior sobre o acretismo em pacientes com placenta prévia. Rev Bras Ginecol Obstet. 1997;19:105—9. 5. Elito Jr J, Montenegro NAMM, Soares RC, et al. Gravidez ectópica não rota — diagnóstico e tratamento. Situação atual. Rev Bras Ginecol Obstet. 2008;30:149—59. 6. Jurkovic D, Hillaby K, Woelfer B, et al. First-trimester diagnosis and management of pregnancies implanted into the lower uterine segment Cesarean section scar. Ultrasound Obstet Gynecol. 2003;21:220—7. 7. Fabres C, Aviles G, De La Jara C, et al. The cesarean delivery scar pouch: clinical implications and diagnostic correlation between transvaginal sonography and hysteroscopy. J Ultrasound Med. 2003;22:695—700. 8. Monteagudo A, Carreno C, Timor-Tritsch IE. Saline infusion sonohysterography in nonpregnant women with previous cesarean delivery: the “niche” in the scar. J Ultrasound Med. 2001;20:1105—15. 9. Thurmond AS, Harvey WJ, Smith SA. Cesarean section scar as a cause of abnormal vaginal bleeding: diagnosis by sonohysterography. J Ultrasound Med. 1999;18:13—6. 10. Torloni MR, Vedmedovska N, Merialdi M, et al. Safety of ultrasonography in pregnancy: WHO systematic review of the literature and meta-analysis. Ultrasound Obstet Gynecol. 2009;33:599—608. 11. Armstrong V, Hansen WF, Van Voorhis BJ, et al. Detection of cesarean scars by transvaginal ultrasound. Obstet Gynecol. 2003;101:61—5. 12. Santos Filho OO, Nardozza LM, Araujo Júnior E, et al. Cesarean uterine scar evaluation by the grey-level histogram. Rev Assoc Med Bras. 2010;56:99—102. 13. Oosterhof H, Aarnoudse JG. Ultrasound pulsed Doppler studies of the uteroplacental circulation: the influence of sampling site and placenta implantation. Gynecol Obstet Invest. 1992;33:75—9. 14. Mäkikallio K, Tekay A, Jouppila P. Uteroplacental hemodynamics during early human pregnancy: a longitudinal study. Gynecol Obstet Invest. 2004;58:49—54. 15. Martin JA, Hamilton BE, Sutton PD, et al. Births: final data for 2002. Natl Vital Stat Rep [serial on the Internet]. 2003[cited2006Oct12];52(10). Available from: http://www.cdc.gov/nchs/data/nvsr/nvsr52/nvsr52_10.2006 16. Albaiges G, Missfelder-Lobos H, Parra M, et al. Comparison of color Doppler uterine artery indices in a population at high risk for adverse outcome at 24 weeks’ gestation. Ultrasound Obstet Gynecol. 2003;21:170—3. 17. Harrington KF, Campbell S, Bewley S, et al. Doppler velocimetry studies of the uterine artery in the early prediction of pre-eclampsia and intra-uterine growth retardation. Eur J Obstet Gynecol Reprod Biol. 1991;42Suppl:S14—20. 18. Montenegro CAB, Lima MLA, Rezende Filho J. Diagnóstico pré-natal: prevenção da toxemia & do parto prematuro. Femina. 2001;29:31—4. 19. Guidoni RG, Toledo SF, Saito M, et al. Avaliação anatomopatológica de cicatrizes uterinas de acordo com o tipo de sutura cirúrgica (modelo experimental). Rev Bras Ginecol Obstet. 2007;29:633—8. 20. Bewley S, Campbell S, Cooper D. Uteroplacental Doppler flow velocity waveforms in the second trimester. A complex circulation. Br J Obstet Gynaecol. 1989;96:1040—6. 21. Schulman H, Fleischer A, Farmakides G, et al. Development of uterine artery compliance in pregnancy as detected by Doppler ultrasound. Am J Obstet Gynecol. 1986;155:1031—6. 22. Faúndes A, Cecatti JG. Cesarean section in Brazil: incidence, trends, causes, consequences and suggestions for change. Cad Saude Publica. 1991;7:150—73. 23. Rotas MA, Haberman S, Levgur M. Cesarean scar ectopic pregnancies: etiology, diagnosis, and management. Obstet Gynecol. 2006;107:1373—81. 24. Zimmer EZ, Bardin R, Tamir A, et al. Sonographic imaging of cervical scars after Cesarean section. Ultrasound Obstet Gynecol. 2004;23:594—8. 1. PhD of Sciences, Professor at Department of Gynecology and Obstetrics – Pontifícia Universidade Católica de Campinas (PUC-Campinas), Campinas, SP, Brazil. 2. Private Docent, Associate Professor, Department of Obstetrics – Universidade Federal de São Paulo (Unifesp), São Paulo, SP, Brazil. 3. PhD, Associate Professor, Department of Obstetrics – Universidade Federal de São Paulo (Unifesp), São Paulo, SP, Brazil. 4. Private Docents, Titular Professors, Department of Obstetrics – Universidade Federal de São Paulo (Unifesp), São Paulo, SP, Brazil. Mailing Address: Dr. Edward Araujo Júnior Departamento de Obstetrícia da Universidade Federal de São Paulo (Unifesp) Rua Napoleão de Barros, 875, Vila Clementino São Paulo, SP, Brazil, 04024-002 E-mail: araujojred@terra.com.br Received February 28, 2011. Accepted after revision April 18, 2011. Study developed at Department of Obstetrics – Universidade Federal de São Paulo (Unifesp), São Paulo, SP, Brazil. |
|
Av. Paulista, 37 - 7° andar - Conj. 71 - CEP 01311-902 - São Paulo - SP - Brazil - Phone: (11) 3372-4544 - Fax: (11) 3372-4554