Radiologia Brasileira - Publicação Científica Oficial do Colégio Brasileiro de Radiologia
AMB - Associação Médica Brasileira CNA - Comissão Nacional de Acreditação
 Vol. 44 nº 2 - Mar. / Apr. of 2011
Vol. 44 nº 2 - Mar. / Apr. of 2011
|
ORIGINAL ARTICLE
|
|
Computed tomography-guided core-needle biopsy of lung lesions: an oncology center experience |
|
|
Autho(rs): Marcos Duarte Guimarães1; Alexandre Calábria da Fonte2; Marcony Queiroz de Andrade3; Rubens Chojniak4; Jefferson Luiz Gross5 |
|
|
Keywords: Needle; Biopsy; Lung; Cancer; Computed tomography. |
|
|
Abstract: INTRODUCTION
Percutaneous biopsies of pulmonary lesions have been performed for over a century( 1). The development of imaging methods, particularly computed tomography (CT), has contributed for a more accurate localization of pulmonary lesions. Likewise, the introduction and improvements of specific needles have contributed to make the procedure universally known and performed on a large scale. Such procedure has actually become a viable alternative to other diagnostic procedures such as sputum cytology, bronchoscopy and thoracotomy, with high rates of appropriateness of material collected for analysis(2,3). However, in the case of benign lesions, the accuracy of such method is lower than that with malignant lesions and, considering the difficulty in maintaining a pathologist on duty in the department of radiology, it was detected the necessity of performing biopsies to provide appropriate material for analysis and specific diagnosis(4). Computed tomographyguided core-needle biopsy of lung lesions has been widely accepted as an effective and safe procedure for specific diagnosis( 5,6). By means of such a procedure, tissue fragments are collected for histological analysis in order to determine the specific nature of lung lesions. It is a simple technique to guide therapeutic decisions, avoiding surgical biopsies in many clinical conditions, and demonstrating to be a viable alternative to fine-needle aspiration (FNA)(7,8). The present study is aimed at describing the experience of an oncology center with CT-guided core-needle biopsy of lung lesions for the collection of appropriate material for analysis and specific diagnosis whenever possible. MATERIALS AND METHODS The present study reports a retrospective analysis of 94 patients admitted to a Brazilian reference oncology center (Hospital do Câncer – A.C. Camargo) and submitted to 97 CT-guided core-needle biopsies of lung lesions in the period from 1996 to 2004. Along this same period, a total of 459 CT-guided lung biopsies were performed, with 362 (78.9%) being FNA and 97 (21.1%) core-needle biopsies. Information on the specimens’ appropriateness and diagnoses specificity were collected from the records at the hospital’s division of medical archives. A term of free and informed consent was signed by all the patients included in the present study. The biopsies were performed with the standard technique, under guidance of axial CT (Pace Plus and Pro Speed; General Electric Medical Systems, Milwaukee, WI, USA). All the patients were submitted to panoramic CT images acquisition (scout), with sections ranging between 5 and 10 mm, for appropriate localization of the lesions and comparison with previous images. The needle was inserted with the patients in expiratory apnea, and new CT scans were performed in order to assure the accurate positioning of the needle in relation to the lesion. An automated coaxial Temno Biopsy 18 gauge (needle and cannula) was utilized in 52 biopsies. After the needle was advanced through the skin with the stylet contained within the cannula, the system was positioned at the margin of the lesion and the location was confirmed by means of CT scans. The stylet was then removed from the cannula and the needle was inserted and triggered, with its cutting tip advancing 20 mm. A 20-gauge needle (MD Tech Tru-Core) was utilized in 45 biopsies. The automated system was engaged and then triggered so that the needle cutting tip advanced 15 or 20 mm, depending on the size of the lesion. The biopsy site could be visualized on the guiding CT images. The cursor on the computer screen was utilized to measure the lesions dimensions in their distances from the metal marker placed on the skin surface. The exact site for the needle insertion into the skin was obtained by means of surface markings made with a surgical pen, correlating the metal marker and the laser beam incident upon the patient with the axial tomographic sections of the lesion, besides the physician’s experience. Such data allowed the calculation of the point of insertion of the needle into the skin, the required needle angle and course and the depth required to reach the lesion. After the marking, a local anesthetic with 1% lidocaine was applied, and the needle was inserted. A guiding CT scan was performed to confirm or correct the needle positioning in relation to the lesion (Figures 1 to 7). In both cases, the procedure was repeated until the specimens were macroscopically appropriate or when pneumothorax occurred. 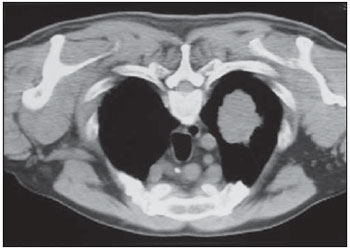 Figure 1. Patient positioned on the table of the CT equipment, according to the location of the lesion. In this case, a lesion on posterior region of the right upper lobe, the most appropriate patient’s positioning for the approach was ventral decubitus. 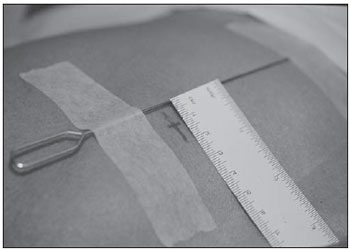 Figure 2. A metal marker is placed on the skin surface and held in position with micropore surgical tape, for the definition of the point of insertion of the needle on a parallel plane and closest to the lesion whenever possible. A millimetric ruler is utilized for the comparison of the measurement performed at the puncture point with the measurement performed on the computer screen. 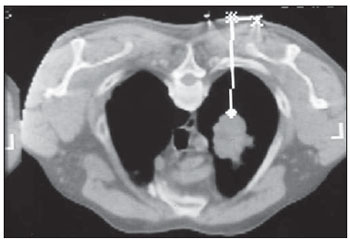 Figure 3. After the confirmation of the similarity between the needle course and the distance measured on the computer screen and on the patient’s skin surface, the measurement of the depth required for the needle to reach the lesion and collect the specimen is performed. 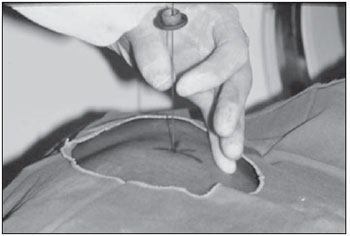 Figure 4. After asepsis, anesthetic is locally applied and also at the deeper planes of the chest walls. The biopsy needle is inserted on the exact site of the skin surface, following the course and distance determined by the analysis performed on the computer. 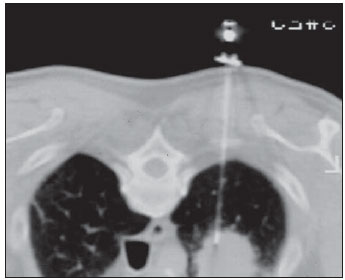 Figure 5. Once the appropriate positioning of the needle within the lesion is confirmed, the specimen collection is performed. 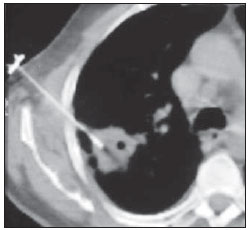 Figure 6. An example of lung lesion located in the posterior region of the upper right lobe. It was decided that the patient would be placed in dorsal decubitus. The closest point to the lesion selected for needle insertion was on the anterior axillary line, avoiding the scapula, and following a diagonal and posterior course in order to reach the center of the lesion. 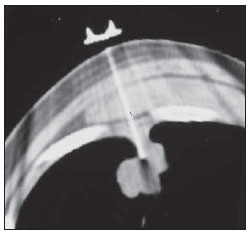 Figure 7. An example of lateral lung lesion approached through a course covering the shortest distance possible between the skin surface and the lesion, with the patient positioned in lateral decubitus. The collected specimens were preserved in 10% formalin and submitted to histological examination. In the present study, most of the specific diagnoses were confirmed by the association of the results from histological analyses of the specimens collected by percutaneous biopsies with the clinical follow-up in the authors’ institution. In the clinical follow-up of the malignant lesions, the diagnosis based on the lung biopsy was considered within the context related to the natural history of the baseline disease, expected therapeutic response criteria and disease progression in spite of instituted treatment. In the clinical follow-up of the benign lesions, the regression of the lesions after specific treatment or radiological stability was considered after at least two years of follow-up. In the cases of divergence between biopsy results and clinical suspicion, the patients were referred for surgical excision of the lesion. Descriptive statistics were utilized to describe the present series. RESULTS Among the 97 core-needle biopsies of lung lesions, 94 (96.9%) produced appropriate specimens for histological analysis, resulting in 71 (73.2%) malignant lesions and 23 (23.7%) benign lesions. Three (3.1%) of the biopsies did not produce appropriate material for analysis. The frequency of specific diagnosis was 83 (85.6%) cases, demonstrating high rates for both malignant lesions [63 cases (88.7%)], and benign lesions [20 cases (86.7%)]. In 83 (85.6%) of the 97 percutaneous biopsies, it was possible to confirm a specific diagnosis, including 78 (80.4%) specific diagnoses confirmed by histological analysis and clinical follow-up, and five (5.2%) specific diagnoses confirmed by surgical resection of the lesion. In 11 (11.3%) biopsies the confirmation of specific diagnosis was not possible as the patients were no longer being followed-up at the institution. In 3 cases (3.1%), the specimens were not appropriate for analysis and consequently the diagnoses could not be achieved. In the group of malignant lesions there were 44 (61.2%) bronchogenic lesions, 23 (32.4%) metastatic lesions and 4 (6.4%) nonspecific malignant lesions. Among the 23 metastatic lesions, 21 specific diagnoses were found, with 14 (60.9%) carcinomas, 4 (17.4%) sarcomas and 3 (13%) lymphomas. In 2 (8.7%) cases, the collected specimens were appropriate for the malignancy diagnosis; however, such specimens were inappropriate for the definition of specific diagnoses. Among the 44 biopsies that led to the diagnosis of bronchogenic carcinomas, 38 (86.4%) corresponded to non-small cell carcinomas [24 (54.5%) adenocarcinomas and 14 (31.9%) epidermoid carcinomas]. In 2 cases (4.5%) the specific diagnoses were small cell carcinomas, and in 4 (10.5%), the type of bronchogenic carcinoma could not be identified. Among the 23 benign lesions biopsies, 20 (86.7%) cases could confirm specific diagnoses, including 15 (65.2%) specific diagnoses confirmed by histological analysis of the specimens and clinical follow-up, and 5 (21.7%) specific diagnoses confirmed by surgical resection of the lesions. In 3 (13.3%) biopsies a specific diagnosis could not be confirmed as the patients were no longer being followed up at the institution and the collected specimens were considered negative for malignancy according to histological analyses, as demonstrated on Table 1. 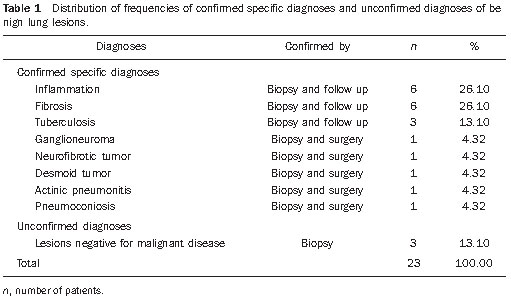 Among the total of 97 core-needle biopsies, 12 (12.4%) cases presented complications, 7 (7.2%) hematomas, 3 (3.1%) pneumothorax, and 2 (2.1%) hemoptysis. In spite of the occurrence of complications, there was neither need for chest drain insertion nor hospitalization (Figure 8). 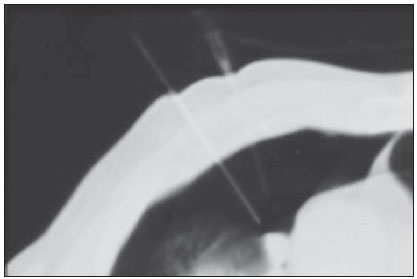 Figure 8. After collection of the specimen, a follow-up CT scan is performed to verify the occurrence of complications. Pneumothorax is one of the most frequent and feared complications. Note a deeply located nodule and the needle tip at 0.5 cm from the nodule. In this case a Jelco 14 gauge relief catheter was inserted into the pleural space filled with air (pneumothorax). The pneumothorax was controlled and the procedure was normally concluded. Chest drainage was not necessary and the patient was discharged four hours after the procedure. DISCUSSION Percutaneous CT-guided core-needle biopsy of lung lesions is a relatively safe method for the diagnosis of benign and malignant lesions(5,6,8–10). Over the last decades the techniques were improved and several studies have evidenced the advantages of such procedure, also demonstrating acceptable rates of incidence of complication and excellent diagnostic results as compared with FNA(1,5,6,8,11,12). Under ideal conditions, every percutaneous image-guided procedure should be assisted by a pathologist for immediate analysis of the collected specimen, defining its quality and the specific diagnosis whenever possible. This would certainly contribute for reducing the number of punctures enhancing the results, and would consequently reduce the procedure-related morbidity. However, for logistic reasons, including availability of space in the CT room, routine of the pathological anatomy department, availability of a pathologist as a routine in the imaging unit and cost considerations, few centers are able to meet the above mentioned conditions, and percutaneous procedures are frequently performed without the presence of a pathologist. In the absence of such professional, the collection of a tissue specimen from a lung lesion by means of core-needle biopsy provides appropriate material for analysis capable of defining a specific diagnosis both in cases of benign and malignant lesions, with possibility of even determining the cellular type of lesion in patients diagnosed with carcinoma(9,13–15). According to the literature, the rate of success in the collection of appropriate specimens of lung lesions by means of FNA ranges from 64.6% to 96.6%(3,16–18). Most recently, studies have demonstrated greater safety guarantees and better diagnostic performance obtained with automated core-needle biopsy systems, and thus the utilization of such procedure is gaining terrain, particularly in cases where one is seeking a specific diagnosis of a benign or malignant disease(7,9,11–14). However, FNA is still a relevant method as some factors are taken into consideration. Among such factors, lower complexity and swiftness in performance, particularly for ill patients with coagulation abnormalities or with difficulties in maintaining the required decubitus and apnea during the procedure. Some studies demonstrate high indices of diagnosis with FNA, particularly when one considers only the need to document the malignant disease, and not the need for a specific diagnosis to make a decision on the therapeutic approach(1,3,4,16–18). Klein et al.(9) have demonstrated global accuracy of 88%, with 95% sensitivity and 91% specificity for the diagnosis of malignancy by means of core-needle biopsy. Lucidarme et al.(14) have demonstrated global diagnostic accuracy of 88% with the utilization of core-needles in the diagnosis of both benign and malignant lesions. Laurent et al.(19) have compared FNA with to CT-guided core-needle biopsies in the diagnosis of malignant lung lesions, and found methods sensitivity of 88% and 97.4%, respectively, demonstrating a statistically significant difference. Yu et al.(5) have demonstrated that core needles, either with or without the coaxial system, provide appropriate specimens (98.1%) for histological analysis and specific diagnosis, with an accuracy of 97.2%, sensitivity of 96.8% and specificity of 100% in the identification of malignant lesions. Chojniak et al.(6) have demonstrated that for lung lesions, the rates of specimens’ appropriateness and diagnosis specificity were always better with coreneedle biopsies than with FNA. With the exception of the three lesions submitted to biopsies whose specimens were not appropriate for analysis, the present study demonstrated high indices of specimens’ appropriateness, with 96.9% of the total sample, and also high indices of diagnoses specificity, both for benign and malignant lesions, with 86.9% and 88.7%, respectively. Such results are similar to those from other studies described in the literature(11,15). Chojniak et al.(6) and Guimarães et al.(18) have demonstrated rates of complication corresponding to 16% and 11.1%, and chest drainage to 4.9% and 3.0%, respectively, in patients submitted to CT-guided FNA of lung lesions. Khan et al.(20), in a study approaching factors predictive factors of complications in core-needle biopsies, have demonstrated a pneumothorax rate of 17% and chest drainage rate of 2%. Carazzai et al.(21), analyzing FNA and CT-guided core-needle biopsies of lung lesions, found a 28.6% global rate of complications (6% corresponding to hematomas with no major implication, and 22.6% corresponding to pneumothorax, two of such cases submitted to aspiration by means of a Jelco 14 gauge catheter inserted into the second intercostal space at the hemiclavicular line, with no need for later chest drainage). In the present study, only core-needle biopsies were evaluated. The total rate of complications was 12.4% (7.2% corresponding to hematomas, 2.1%, hemoptysis, and 3.1% related to pneumothorax), without the need for chest drainage or hospitalization, which can be considered a reduced rate as compared with other rates reported in the literature(4,9,13,14,20–22). The Hospital A.C. Camargo Department of Radiology has routinely been performing CT-guided percutaneous transthoracic procedures, and over the last ten years has become a reference center(3,5,6,18,23,24). The high performance of core-needle biopsy can be partially explained by the selection of different needle types and sizes, according to the lesions characteristics. For the lungs, there is a premise regarding the use of fine needles in smaller, deeper and probably malignant lesions, and thicker needles in larger and superficial lesions in case of doubts regarding their benign nature. Perhaps the experience acquired over the past years with such procedures may have contributed to minimize the occurrence of complications in the present study sample. However, because of the retrospective nature of the present study, there are limitations that must be considered, particularly regarding the possibility of occurrence of selection bias and also the possibility of underestimation of complication rates. Based on the results of the present study, reports in the literature and on the experience acquired in this procedure for over 15 years at the Department of Radiology of the A.C. Camargo Hospital, core needles are currently utilized for most lung biopsies, while the fine needles are only utilized in severely ill patients, patients with coagulation abnormalities, uncooperative patients or those requiring only the confirmation of malignancy for decision making about the therapeutic approach. CONCLUSION In the present study, percutaneous CTguided core-needle biopsy of lung lesions demonstrated high rates of specimens’ appropriateness and diagnoses specificity, and reduced rates of complications. REFERENCES 1. Zavala DC, Schoell JE. Ultrathin needle aspiration of the lung in infectious and malignant disease. Am Rev Respir Dis. 1981;123:125–31. 2. Junqueira MAF, Camara-Lopes LH, Albertotti C, et al. Biópsia pulmonar transtorácica orientada por tomografia computadorizada. J Pneumol. 1990;16:1–5. 3. Guimarães MD, Chojniak R, Gross JL, et al. Predictive success factors for CT-guided fine needle aspiration biopsy of pulmonary lesions. Clinics. 2009;64:1139–44. 4. Küçük CU, Yilmaz A, Yilmaz A, et al. Computed tomography-guided transthoracic fine-needle aspiration in diagnosis of lung cancer: a comparison of single-pass needle and multiple-pass coaxial needle systems and the value of immediate cytological assessment. Respirology. 2004;9: 392–6. 5. Yu LS, Deheinzelin D, Younes RN, et al. Computed tomography-guided cutting needle biopsy of pulmonary lesions. Rev Hosp Clin Fac Med Sao Paulo. 2002;57:15–8. 6. Chojniak R, Isberner RK, Viana LM, et al. Computed tomography guided needle biopsy: experience from 1,300 procedures. Sao Paulo Med J. 2006;124:10–4. 7. Moulton JS, Moore PT. Coaxial percutaneous biopsy technique with automated biopsy devices: value in improving accuracy and negative predictive value. Radiology. 1993;186:515–22. 8. Haaga JR, Alfidi RJ, Zelch MG, et al. Computed tomography of the pancreas. Radiology. 1976; 120:589–95. 9. Klein JS, Salomon G, Stewart EA. Transthoracic needle biopsy with a coaxially placed 20-gauge automated cutting needle: results in 122 patients. Radiology 1996;198:715–20. 10. Westcott JL, Rao N, Colley DP. Transthoracic needle biopsy of small pulmonary nodules. Radiology. 1997;202:97–103. 11. Boiselle PM, Shepard JAO, Mark EJ, et al. Routine addition of an automated biopsy device to fine needle aspiration of the lung: a prospective assessment. AJR Am J Roentgenol. 1997;169:661–6. 12. Arakawa H, Nakajima Y, Kurihara Y, et al. CTguided transthoracic needle biopsy: a comparison between automated biopsy gun and fine needle aspiration. Clin Radiol. 1996;51:503–6. 13. Haramati LB. CT-guided automated needle biopsy of the chest. AJR Am J Roentgenol. 1995; 165:53–5. 14. Lucidarme O, Howarth N, Finet JF, et al. Intrapulmonary lesions: percutaneous automated biopsy with a detachable,18-gauge, coaxial cutting needle. Radiology. 1998;207:759–65. 15. Horrigan TP, Bergin KT, Snow N. Correlation between needle biopsy of lung tumors and histopathologic analysis of resected specimens. Chest. 1986;90:638–40. 16. Shimizu K, Ikeda N, Tsuboi M, et al. Percutaneous CT-guided fine needle aspiration for lung cancer smaller than 2 cm and revealed by ground-glass opacity at CT. Lung Cancer. 2006;51:173–9. 17. Stanley JH, Fish GD, Andriole JG, et al. Lung lesions: cytologic diagnosis by fine-needle biopsy. Radiology. 1987;162:389–91. 18. Guimarães MD, Andrade MQ, Fonte AC, et al. Predictive complication factors for CT-guided fine needle aspiration biopsy of pulmonary lesions. Clinics. 2010;65:847–50. 19. Laurent F, Latrabe V, Vergier B, et al. Percutaneous CT-guided biopsy of the lung: comparison between aspiration and automated cutting needles using a coaxial technique. Cardiovasc Intervent Radiol. 2000;23:266–72. 20. Khan MF, Straub R, Moghaddam SR, et al. Variables affecting the risk of pneumothorax and intrapulmonal hemorrhage in CT-guided transthoracic biopsy. Eur Radiol. 2008;18:1356–63. 21. Carazzai EH, Andreosi M, Gonzalez FM, et al. Biópsia pulmonary percutânea guiada por tomografia computadorizada: dados de um hospital. Radiol Bras. 2006;39:277–82. 22. Yeow KM, See LC, Lui KW, et al. Risk factors for pneumothorax and bleeding after CT-guided percutaneous coaxial cutting needle biopsy of lung lesions. J Vasc Interv Radiol. 2001;12:1305– 12. 23. de Farias AP, Deheinzelin D, Younes RN, et al. Computed tomography-guided biopsy of mediastinal lesions: fine versus cutting needles. Rev Hosp Clin Fac Med Sao Paulo. 2003;58:69–74. 24. Guimaraes MD, de Andrade MQ, da Fonte AC, et al. CT-guided cutting needle biopsy of lung lesions – an effective procedure for adequate material and specific diagnose. Eur J Radiol. 2010; 26. [Epub ahead of print]. 1. Fellow PhD degree in Sciences, Preceptor of Medical Residency in Radiology and Imaging Diagnosis at Hospital A.C. Camargo and Hospital Heliópolis, São Paulo, SP, Brazil. 2. Master, MD, Radiologist Assistant, Hospital A.C. Camargo, São Paulo, SP, Brazil. 3. MD, Radiologist, Hospital Aliança, Salvador, BA, Brazil. 4. PhD, Director of Department of Radiology and Imaging Diagnosis, Hospital A.C. Camargo, São Paulo, SP, Brazil. 5. PhD, Director of Department of Chest Surgery, Hospital A.C. Camargo, São Paulo, SP, Brazil. Mailing Address: Dr. Marcos Duarte Guimarães Hospital A.C. Camargo – Departamento de Radiologia e Diagnóstico por Imagem Rua Antônio Prudente, 211, Liberdade São Paulo, SP, Brazil, 01509-010 E-mail:marcosduarte@yahoo.com.br Received September 18, 2010. Accepted after revision March 31, 2011. Study developed at Hospital A.C. Camargo – Fundação Antônio Prudente, São Paulo, SP, Brazil. |
|
Av. Paulista, 37 - 7° andar - Conj. 71 - CEP 01311-902 - São Paulo - SP - Brazil - Phone: (11) 3372-4544 - Fax: (11) 3372-4554