INTRODUCTION
Ultrasonography is an important tool in the evaluation of adnexal masses and in the definition of differential diagnosis for such conditions(1). In children, because of the limitations of clinical examination, abdominal ultrasonography becomes a preferential method in the complementary investigation(2). Transvaginal ultrasonography (TVUS) is currently considered as a highly accurate method in the evaluation of adnexal masses and ovarian cysts(1–3).
This diagnostic modality has been directly related to the operator ability and experience, particularly in the differential diagnosis between malignant and benign adnexal masses, although several authors have reported similar results demonstrating a high diagnostic accuracy of the method(3–9). The operator must be familiar with all possible presentations of a normal ovary as well as with the characteristics of probably benign or malignant lesions.
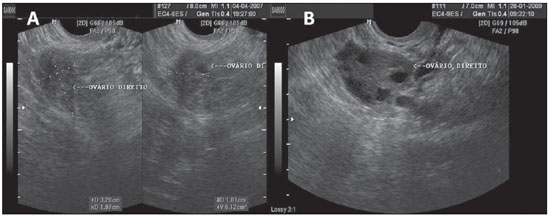
Figure 1.
Normal ovaries. A,B: TVUS showing morphologically normal ovaries. On A, technique for measuring ovarian volume.
Tridimensional ultrasonography and Doppler as well as tumor markers, namely, CA-125, CA-15.3, CA-19.9, CA-72.4 and alpha-fetoprotein blood tests are recommended as adjuvant methods in the differential diagnosis of adnexal masses, considering that they increase the sensitivity and specificity in the differentiation of ovarian tumors(9–12).
With a view on the challenges of differential diagnosis as well as the possibility of morphological suspicion of ovarian neoplasias at ultrasonography, the present article provides a brief description of the most common presentations of adnexal masses.
FUNCTIONAL CYSTS
Among the cystic adnexal masses, functional cysts resulting from the normal ovarian function are the most common ones, but their actual incidence is still to be known because in most of cases such cysts are asymptomatic (Figure 2)(1,7).
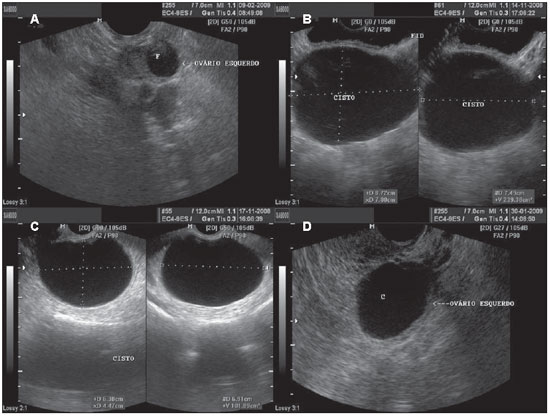
Figure 2.
Functional cyst. A: TVUS demonstrating left ovary with a dominant follicle (F). B: Abdominal ultrasonography demonstrating a simple cyst, with anechoic fluid content, with defined contour and thin walls. C,D: Images depicting the same characteristics, with TVUS of different patients.
Follicular cysts are frequently observed during menacme and may occur in up to 17% of postmenopausal women. Classically, such cysts present with unilocular aspect, thin walls and may achieve up to 8 cm in diameter(1,13). Frequently anechoic fluid serous content is observed, with the possibility of complicating with hemorrhage(13).
Corpus luteum cysts are frequently found in the first trimester of gestation, commonly achieving the maximum size at the tenth week, with spontaneous regression around the 16th week of gestation. Corpus luteum cysts are also a usual finding in the second phase of the menstrual cycle in non-pregnant women. Their typical sonographic aspect consists of findings of cystic mass with echogenic walls and occasional hyperechogenic contents in the cases of hemorrhagic cysts (Figure 3)(7).
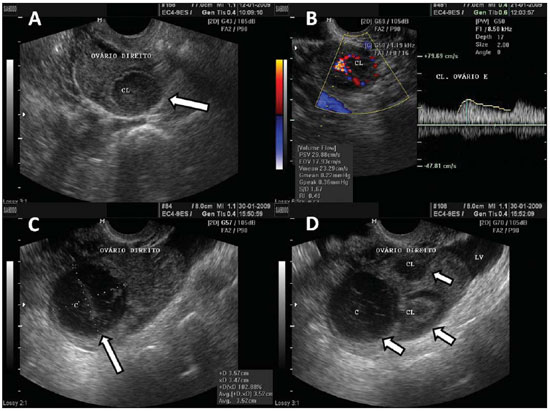
Figure 3.
Corpus luteum. A: Right ovary with a cystic, thin-walled mass in its interior, with echogenic content, characteristic of corpus luteum (arrow). B: Color Doppler – the halo of vascularization around the corpus luteum (CL) characterizes the “ring-of-fire” image pattern. C: Right ovary with corpus luteum (arrow) with debris suggestive of hemorrhage in its interior – heterogeneous linear echogenic content with a reticular aspect. D: Right ovary with three corpus lutea (arrows) and free peritoneal fluid (LV) in a patient submitted to ovulation induction therapy.
Theca-lutein cysts are usually large, multiloculated and bilateral, and result from hormonal stimulation by high circulating levels of hCG. Such cysts may be found in cases of gestational trophoblastic disorder, multiple gestation, or in the condition known as
hyperreactio luteinalis(13).
Hemorrhagic cysts (Figure 4) are most frequently observed in premenopausal women and may present with pelvic pain, typically at mid-cycle(7). Characteristically, they present with a linear heterogeneous echographic pattern in several planes. Blood clots (Figure 5) may be demonstrated as echogenic, heterogeneous masses within the cyst, with no sign of vascularization at Color Doppler, sometimes with images suggesting neoplasia(7,13). Serial follow-up is suggested for such patients, as such cysts usually disappear within eight weeks(7,13).
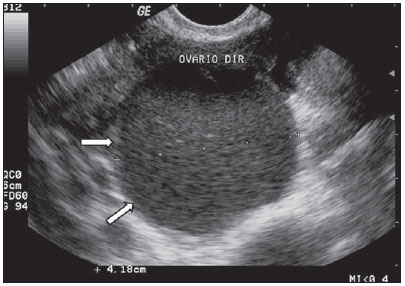
Figure 4.
Hemorrhagic cyst. TVUS demonstrating well delimitated cyst-like mass (arrows), with linear heterogeneous content in several planes, with a reticular pattern, in the right ovary, compatible with a cyst with hemorrhagic content. Differential diagnosis with endometrioma should be performed in the first phase of the next menstrual cycle.
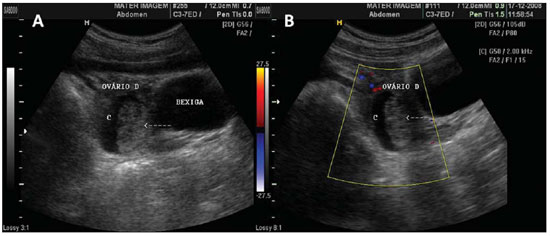
Figure 5.
Blood clot in a hemorrhagic cyst. A,B: Hemorrhagic cyst (C) with a mass with heterogeneous echogenicity in its interior, compatible with retracted clot (arrow) in right ovary at abdominal ultrasonography. B: The use of color Doppler US in the same patient, rules out the presence of vascularization within the mass interior (arrow), supporting the characterization of the clot.
These cysts originate from mesonephric (Wolffian), paramesonephric (Müllerian) structures, or mesothelial inclusions(1). The vesicular appendix (Morgagni hydatid) is the most common type of paramesonephric cyst, frequently found at one of the fimbriae at the end of the uterine tube. Echographically, they present with thin, deformable walls, generally with the largest diameter measuring up to 10 mm, next to a normal ovary but separated from it (Figure 6)(1,7).
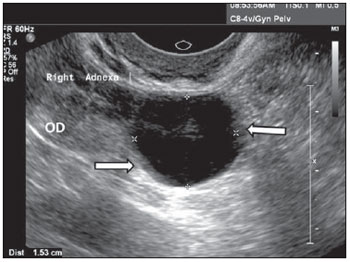
Figure 6.
Paraovarian cyst. Ultrasonography of the right annex, demonstrating the image of a unilocular, thin-walled cyst with anechoic content, adjacent and separated from the ipsilateral ovary (OD). The arrows show subtle internal protrusions of the cyst’s walls.
Peritoneal inclusion cysts classically occur as a result from accumulation of ovarian fluid trapped by peritoneal adhesions in patients with a history of abdominal surgery, trauma, pelvic inflammatory disease or endometriosis. Typically, these are large cystic multiloculated masses, separated from the ovaries, which present with a normal echographic aspect (Figure 7)(7).
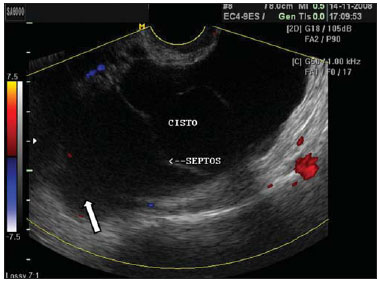
Figure 7.
Peritoneal inclusion cyst. TVUS showing a large cyst-like septate mass with irregular echogenic content (arrow), separated from the ovary, and with no sign of vascularization at color Doppler US. Such cysts may occur at the center of the pelvis in patients previously submitted to pelvic surgery.
Endometriomas are homogeneous and well delimited structures, with low to median echogenic density contents, and may commonly present septations. Presentations with hyperechoic walls or nodulations(13–15) are described. Endometriomas may present with an avascular calcified solid component, with the presence of posterior attenuation or acoustic shadow. In the presence of solid or mixed components they may be confused with hemorrhagic cysts or neoplasia, but differ from these because of the more homogeneous echogenic pattern (Figure 8)(1,13–15).
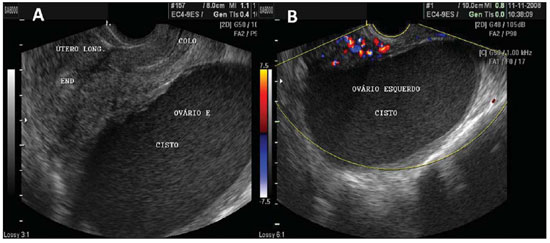
Figure 8.
Endometriomas. A: Endometrioma in left ovary – cystic mass filled by low intensity echoes, with more homogeneous pattern than hemorrhagic cysts and with well delimited walls. B: The same endometrioma, with visualization at color Doppler US, showing absence of vessels in its interior, thus ruling out the presence of a solid mass.
Fibromas are the most common benign solid neoplasias of the ovaries. They may be found at any age, however their incidence is higher in middle-aged patients(14). At ultrasonography they are visualized as solid, hypoechoic and homogeneous images, with the possibility of presenting with acoustic beam attenuation. Dense calcifications may be observed in fibromas, and may be visualized as an posterior acoustic shadowing (Figure 9)(14,16,17).
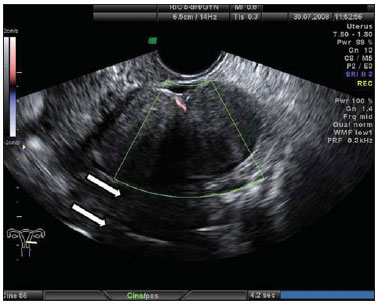
Figure 9.
Ovarian fibroma. TVUS showing a solid hypoechogenic, delimited mass, without vascularization halos at color Doppler US, and with posterior acoustic shadow (arrows).
The association of ovarian fibroma, ascites and hydrothorax characterizes Meigs syndrome(14).
EPITHELIAL SURFACE TUMORS
Epithelial surface tumors comprise approximately 60% of all ovarian neoplasms and up to 90% of primary ovary neoplasms(1).
Serous cystadenomas comprise approximately 20% of the benign ovarian masses. They present as complex thin-walled, uni- or multilocular cysts, with variable sizes, sometimes achieving more than 20 cm. The image of its interior shows echogenic contents, possibly also revealing areas of papillary projections (Figure 10)(13,14,16,17).
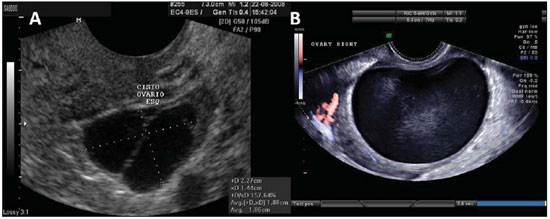
Figure 10.
Serous cystadenoma. A: TVUS of left ovary demonstrating serous cystadenoma with fine septation. B: TVUS demonstrating a unilocular mass with anechoic content, with well delimited wall, that may achieve a large volume characteristic of serous cystadenoma. The finding of minute papillary projection from the wall of the serous cystadenoma is frequent.
Mucinous cystadenomas represent up to 25% of all ovarian neoplasias(1). Usually, ultrasonography reveals echogenic, thin walled multiloculated cystic mass, with echographic content that may vary according to the presence of variable amounts of blood or protein in its interior. Such possible differences in density of the cystic content confer various echogenic presentations in multiple compartments(14,16). The presence of variable echogenic contents in a multiloculated adnexal cystic mass is suggestive of mucinous cystadenoma (Figure 11)(14).
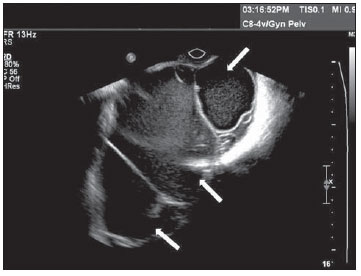
Figure 11.
Mucinous cystadenoma. TVUS of ovary demonstrating mucinous cystadenoma – a multiloculated mass with fine and numerous septations, with echogenic content varying among the several compartments of the mass (arrows), a cluster of small cysts and fine echoes resulting from the thick content.
Cystadenocarcinomas represent up to 10% of the primary ovarian neoplasias. They are usually multiloculated, with multiple projections and gross septations. The use of Doppler ultrasonography demonstrates vascularization of the contents (Figure 12). Massive ascites frequently disproportional to the demonstrated adnexal masses is a usual finding(1,7,14,16).
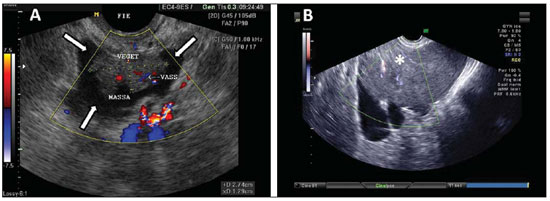
Figure 12.
Cystadenocarcinoma. A: TVUS demonstrating typically suspicious image of malignancy – a heterogeneous mass in the region of the left iliac fossa (arrows) with irregular contour, heterogeneous echogenic content, with Doppler US demonstrating vascularization in the solid component of the mass. B: TVUS also showing an echogenic complex mass, with color Doppler US demonstrating vascularization of the solid component of the mass (asterisk).
Transitional cell tumors, clear cell carcinoma and undifferentiated carcinoma are less frequently found and difficult to differentiate at ultrasonography. Generally, they are unilateral(7).
Endometrioid tumors present both as cystic masses with papillary projections and solid masses in some cases. These tumors are predominantly malignant (approximately 80%), and endometrioid carcinoma is the second most frequently found malignant epithelial ovarian tumor(7). Clear cell carcinoma comprises from 5% to 10% of the malignant ovarian epithelial-stromal tumors, and presents with nonspecific sonographic characteristics, as large complex, generally cystic masses.
Transitional cell tumors (Brenner tumors) represent the minority of the ovarian neoplasias (1.55 to 2.5%) and most of times are benign(7,13). The sonographic pattern of such tumors is characterized by small and solid hypoechoic masses, and sometimes calcifications are identified(1,7).
GERM CELL TUMORS
Mature cystic teratomas of the ovary, or ovarian dermoid cysts, are the most common ovarian neoplasms(1,2).They may be identified in all age groups, however in the childhood and adolescence they are frequent causes of ovarian torsion and may be bilateral in up to 10% of cases(2). Typical sonographic findings are the presence of focal or diffuse high-amplitude echoes, areas with posterior acoustic beam attenuation, and visualization of hyperechogenic lines and spots within the mass (Figure 13). In mature cystic teratomas, such heterogeneous findings translate into the presence of calcified tissues similar to bones and teeth, hair and fat tissue(2,7,14,16,17).
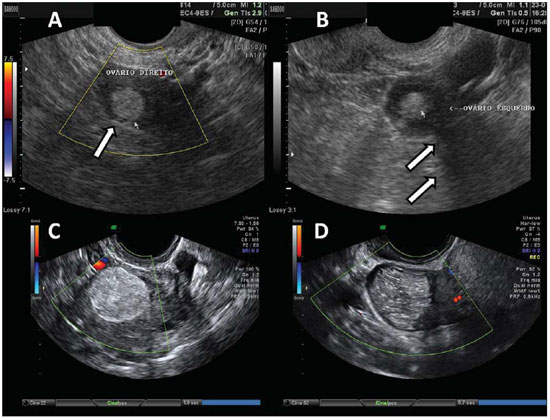
Figure 13.
Teratoma. A: Finding of an echogenic fatty mass, with irregular and heterogeneous content, with linear echoes, and without vascularization halos at color Doppler US, in right ovary (arrow). B: Similar pattern, with the presence of posterior acoustic beam attenuation (arrows) compatible with fatty heterogeneous content with calcifications. C,D: TVUS of different patients – mass with presence of brilliant and diffuse regional echoes, hyperechogenic wall, typically irregular content with echogenic lines and spots, and with no sign of vascularization at color Doppler US.
Ovarian torsion is a gynecological emergency and it is very frequently associated with significant abdominal pain. Mature cystic teratomas and paraovarian cysts are known to be common causes of such torsion(1,2). In children, the excessive mobility of the normal ovary in the pelvis is its main cause(2). At ultrasonography, the more frequent presentation is the increase in size of the affected ovary, normally by more than 4 cm. However, such increase in size may achieve up to 28 times the normal dimensions(18). Ovarian stroma may present with a heterogeneous pattern, with areas of hemorrhage and edema. Complex adnexal or pelvic-abdominal mass with solid or mixed cystic components, as well as the presence of free pelvic fluid is a frequent finding. Color Doppler may demonstrate the absence of vascularization of the affected ovary, however this method is not considered as being a reliable in the evaluation of this condition(2,18). The differential diagnosis is difficult, and can be done with hemorrhagic cyst, endometriosis, ectopic pregnancy or pelvic inflammatory disease(1,7,18).
Tubo-ovarian abscess results from lower genital tract infection that ascends and causes salpingitis (Figure 14) and ovarian inflammation, to the extent of changing the normal morphology of adnexal structures. The sonographic findings depend upon the mass presentation after resolution of the infectious condition. A purely cystic mass with multiple loci, fine septations, or with debris(7,13,15,16) may be visualized. In the abscess, the ovary may not be separately distinguishable from the adnexal structures, while in the tubo-ovarian complex, the ovary is distinguishable from the local inflammatory process(7,13).
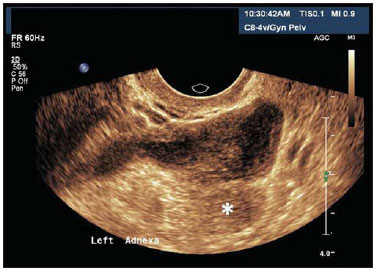
Figure 14.
Salpingitis. TVUS demonstrating an echogenic image with tubular shape, adjacent to the ovary (asterisk) with heterogeneous content in its interior. The patient reported important pain in the left adnexal region during the performance of the examination.
At ultrasonography, hydrosalpinges is characterized by the finding of uterine tube dilation, usually in the segments of the ampulla and infundibulum, showing a tubular and elongated, sometimes serpiginous format, with a clear serous content(7). The presence of incomplete septations and small linear projections are predictors of hydrosalpinges. At ultrasonography, the tubular shape of the mass, and the presence of diametrically opposed protrusions along the mass walls are reliable sonographic markers for the diagnosis (Figure 15)(14,15).
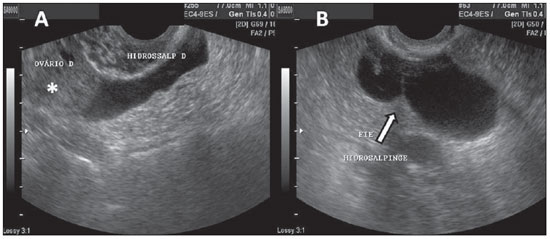
Figure 15.
Hydrosalpinges. A: TVUS showing an anechoic image with tubular shape, adjacent to the ovary (asterisk). B: The arrow indicates the image of incomplete septation with linear projection in an anechoic structure with tubular shape in left iliac fossa, characterizing hydrosalpinges.
The correct sonographic identification of adnexal masses is fundamental both for screening of benign conditions and for the early diagnosis and better follow-up of malignant lesions(2,9,11).
The sonographer must, therefore, be well prepared and aware of the different usual presentations of adnexal masses, in order to be able to interact with the assisting physician with the purpose of proposing strategies that may best guide the specific therapy for the patient.
Acknowledgments
To the PIBIC/CNPq Program, for financing the scholarship for scientific initiation.
REFERENCES
1. Dill-Macky MJ, Atri M. Ultra-sonografia ovariana. In: Callen PW, editor. Ultra-sonografia em obstetrícia e ginecologia. 4ª ed. Rio de Janeiro, RJ: Guanabara Koogan; 2002. p. 803–41.
2. Martins WP, Leite SP, Nastri CO. Ultrassonografia pélvica em crianças e adolescentes. Radiol Bras. 2009;42:395–401.
3. de Kroon CD, van der Sandt HAGM, van Houwelingen JC, et al. Sonographic assessment of non-malignant ovarian cysts: does sonohistology exist? Hum Reprod. 2004;19:2138–43.
4. Murta EFC, Nomelini RS. Early diagnosis and predictors of malignancy of adnexal masses. Curr Opin Obstet Gynecol. 2006;18:14–9.
5. Yazbek J, Raju SK, Ben-Nagi J, et al. Effect of quality of gynaecological ultrasonography on management of patients with suspected ovarian cancer: a randomised controlled trial. Lancet Oncol. 2008;9:124–31.
6. Bagheban AA, Zayeri F, Anaraki FB, et al. The reliability and distinguishability of ultrasound diagnosis of ovarian masses. Indian J Med Sci. 2008;62:217–21.
7. Joshi M, Ganesan K, Minshi HN, et al. Ultrasound of adnexal masses. Semin Ultrasound CT MR. 2008;29:72–97.
8. Valentin L, Ameye L, Testa A, et al. Ultrasound characteristics of different types of adnexal malignancies. Gynecol Oncol. 2006;102:41–8.
9. Fernandes LRA, Lippi UG, Baracat FF. Índice de risco de malignidade para tumores de ovário incorporando idade, ultra-sonografia e o CA-125. Rev Bras Ginecol Obstet. 2003;25:345–51.
10. Reis FJC. Rastreamento e diagnóstico das neoplasias de ovário – papel dos marcadores tumorais. Rev Bras Ginecol Obstet. 2005;27:222–7.
11. Van Calster B, Timmerman D, Bourne T, et al. Discrimination between benign and malignant adnexal masses by specialist ultrasound examination versus serum CA-125. J Natl Cancer Inst. 2007;99:1706–14.
12. Kurjak A, Prka M, Arenas JMB, et al. Three-dimensional ultrasonography and power Doppler in ovarian cancer screening of asymptomatic peri- and postmenopausal women. Croat Med J. 2005;46:757–64.
13. Brown DL. A practical approach to the ultrasound characterization of adnexal masses. Ultrasound Q. 2007;23:87–105.
14. Joshi M, Ganesan K, Munshi HN, et al. Sonography of adnexal masses. Ultrasound Clin. 2008;3:369–89.
15. Kocakoc E, Bhatt S, Dogra VS. Endometriosis. Ultrasound Clin. 2008;3:399–414.
16. Patel MD. Practical approach to the adnexal mass. Radiol Clin North Am. 2006;44:879–99.
17. Jeong YY, Outwater EK, Kang HK. Imaging evaluation of ovarian masses. Radiographics. 2000;20:1445–70.
18. Chang HC, Bhatt S, Dogra VS. Pearls and pitfalls in diagnosis of ovarian torsion. Radiographics. 2008;28:1355–68.
1. MD, graduated at Universidade Estadual do Ceará (UECE), formerly holder of a scholarship of Undergraduate Research – PIBIC/CNPq, Fortaleza, CE, Brazil.
2. PhD, Staff Specialist Obstetrician, The Royal Women’s Hospital, Melbourne, VIC, Australia.
3. PhD, Associate Professor, Division of Gynecology and Obstetrics, Universidade Estadual do Ceará (UECE), Fortaleza, CE, Brazil, Postdoctoral Fellow, Department of Perinatal Medicine, The Royal Women’s Hospital, Melbourne, VIC, Australia.
Mailing Address:
Dr. Fabrício Costa
Ultrasound Department, Royal Women’s Hospital
Locked Bag 300, Grattan St & Flemington Rd
Parkville 3052 VIC, Australia
E-mail: fabricio.costa@thewomens.org.au
Received September 3, 2009.
Accepted after revision July 23, 2010.
 Vol. 44 nº 1 - Jan. /Feb. of 2011
Vol. 44 nº 1 - Jan. /Feb. of 2011














