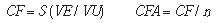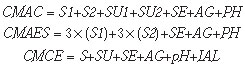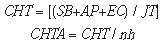INTRODUCTION
The developments occurred since 1960 in the areas of instrumentation and radiopharmaceuticals has determined a remarkable increase in the relevance of nuclear medicine diagnosis methods in the clinical practice nowadays, particularly on account of their accuracy and capacity to deliver an early diagnosis in cases of important diseases such as cancer, neurological and cardiac disorders. Additionally, the utilization of radiopharmaceuticals has gained importance in therapeutic processes with the use of alpha and beta particles emitting radioisotopes.
However, the benefits of the technique can only be achieved when all the agents involved in the process (equipment, radiopharmaceuticals and practitioners) meet high quality standards. For that reason and, considering the risks associated with the use of ionizing radiation, several standards and procedures have been internationally established with respect to the implementation of radiological protection in nuclear medicine centers as well as the quality control of instruments(1–3) and radiopharmaceuticals(4–6).
In Brazil, radiological protection and equipment quality control are already properly regulated(7–9) and under constant supervision. However, for radiopharmaceuticals the situation is quite different – even though this is a crucial matter –, since a majority of radiopharmaceuticals utilized in nuclear medicine clinics is obtained
in situ by reacting sodium pertechnetate (Na[
99mTc]O4) with several chemical reagents contained in a lyophilized
kit, which may lead to the formation of impurities(10).
Problems with the poor efficiency in the labeling of such kits have been reported by several authors(11–13), and performing quality control serves the purposes of preventing inappropriate products from being utilized in patients, as well as unusual image patterns from being correlated with some kind of uncommon disorder, instead of being considered as the result of failures in the radiopharmaceuticals preparation process. In both cases, patients would be submitted to new examinations, with unnecessary radiation exposure. Examples of such cases are the Rotor syndrome and Dubin-Johnson syndrome. Images presented on Figure 1 (obtained from the images database of the Service of Nuclear Medicine of Instituto de Radiologia do Hospital das Clínicas da Faculdade de Medicina da Universidade de São Paulo) show the distribution of the radiopharmaceutical [
99mTc]DISIDA with characteristics that may be confused, respectively, with the administration of [
99mTc]O4– itself or [
99mTc] colloidal technetium, an impurity that may occur during the preparation of [
99mTc]technetium-labeled radiopharmaceuticals.
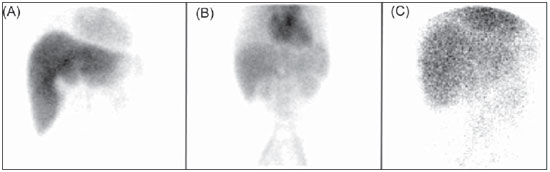
Figure 1. Biliary scintigraphy with the utilization of
99mTc-DISIDA radiopharmaceutical. A: Study within the normality parameters, with radiopharmaceutical presenting radiochemical purity (RCP) = 98.5%. B: Study indicative of Rotor syndrome (RCP = 98.3%). C: Study indicative of Dubin-Johnson syndrome (RPC = 98.7%).
On June 04, 2008, the Brazilian Health Surveillance Agency (Anvisa)(14) issued its resolution RDC No. 38 on the installation and operation of
in vivo nuclear medicine centers. Such resolution instituted the first Brazilian regulation that makes the quality control of generator eluates and radiopharmaceuticals mandatory in nuclear medicine centers. On December 18, 2009 Anvisa issued resolution RDC No. 63, classifying areas of preparation and manipulation of radiopharmaceuticals in nuclear medicine clinics as radiopharmaceutical producing units, and these shall follow the standards established by the mentioned resolution(15).
Considering that radiopharmaceutical quality control requires investment and potentially generates cost increases, the present study describes the results from the analysis on the deployment of a [
99mTc]technetium-labeled generator and radiopharmaceuticals quality control program.
MATERIALS AND METHODS
The cost of the quality control program implementation was based on the costs of all consumable and permanent materials necessary for such implementation, quoted in Brazilian currency or whenever such materials were imported, the considered exchange rate was US$ 1.00 = R$ 1.90.
Permanent material
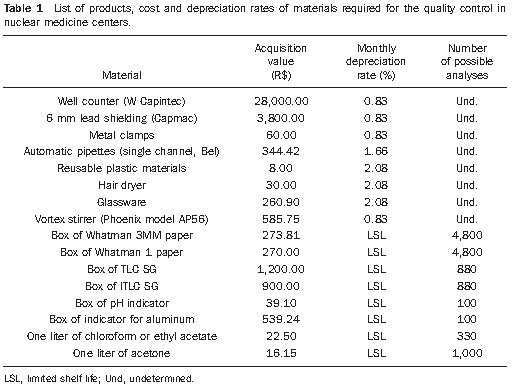
Permanent materials were considered as being those with a useful life of more than 24 months and that can be repeatedly used in several analyses. Thus, well counter-W (Capintec Inc.; Ramsey, NJ, USA), the Capmac 6 mm lead shielding (Capintec Inc.; Ramsey, NJ, USA), the vortex-type mixer and the metal clamps had their useful live estimated in 120 months, while the micropipettes’ as being 60 months, and glassware, hair dryer and plastic materials’ as being 48 months.
Consumables
The following materials were considered as consumables: methanol, ethyl acetate, chloroform solvents, saline solution and the chromatography plates types ITLC-SG (instant thin-layer chromatography-silica gel) (Pall Corporation; New York, NY, USA), TLC-SG (thin-layer chromatography-silica gel) (Merck KgaA; Darmstadt, Germany), Whatman 3MM paper (Whatman International Ltd; Maidstone, England), litmus paper and aluminum indicator (Merck KgaA; Darmstadt, Germany).
Labor
The professionals responsible for the performance of the controls were divided into two groups: the radiology technician, working 96 hours per month and the college graduated professionals, graduated in Pharmacy, Biomedicine or Biology, working 175 hours per month. Besides the professionals’ base salaries, the hazard pay (40% for radiology technicians and 30% for college graduates) and social security and labor taxes (50% of base salary) were calculated in order to determine the total hourly cost of labor.
Calculation formulas
The formulas utilized for determining the costs for deployment of the radiopharmaceuticals quality control program are presented below.
a) Fixed costs
The fixed costs were calculated on the basis of the list of permanent materials, taking their useful lives into account.
where:
CF = fixed cost;
VE = equipment and utensils values;
VU = number of months of the materials useful life;
CFA = fixed cost per analysis;
n = number of analysis per month.
b) Variable costs
The variable costs were determined on the basis of the list of consumables utilized for each analysis, and the amount of such materials utilized in only one individual test. In the present study, one considered the performance of quality control of lipophilic radiopharmaceuticals [
99mTc]MIBI and [
99mTc]ECD by the solvent extraction method(16). For the other radiopharmaceuticals, as well as the radiochemical control of [
99mTc]O4– eluated from the generator, the used method was chromatography on paper or thin layer plates(6).
The formulas for the calculation of cost for each analysis are the following:
where:
CMAC = cost of materials for chromatographic analysis of radiopharmaceuticals;
CMAES = cost of materials for analysis by solvent extraction;
CMCE = cost of materials for control of eluate;
S1 = unit values for solvent 1 (1 ml);
S2 = unit value for solvent 2 (1 ml);
SU = value of the chromatographic support or stationary phase;
SE = value of the syringe;
AG = value of the needle;
pH = unit value of litmus paper;
IAL = unit value of aluminum indicator.
c) Labor costs
The labor costs were determined on the basis of reference salary of professionals graduated in Technological Radiology or with college graduation level in pharmacy, biomedicine or biology, and by incorporating into such salaries other labor rights such as vacations, FGTS (Government Severance Indemnity Fund for Employees) and hazard pay.
where:
CHT = cost of worked hour;
SB = base salary;
AP = hazard pay;
EC = Social security, labor taxes, vacations;
JT = workday hours;
CHTA = cost of worked hour per analysis;
nh = number of analyses per hour.
d) Cost of the analyses
For the calculation of the final cost for each type of analysis, the fixed cost, variable cost and labor cost were summed up, for the respective method. The formulas for the calculation of final quality control cost are the following:
where:
CAC = cost of chromatographic analysis;
CAES = cost of solvent extraction analysis;
CAE = cost of eluate analysis;
CMAC = cost of materials for chromatographic analysis of radiopharmaceuticals;
CMAES = cost of materials for analysis by solvent extraction;
CMCE = cost of materials for control of eluate;
CFA = fixed cost per analysis;
CHTA = cost of worked hour per analysis.
RESULTS
In the calculation of the cost of analysis for quality control, the monthly depreciation costs for the permanent materials were considered according to their monthly depreciation rate. Such depreciation resulted in a monthly value of R$ 273.01. In order to calculate how much the fixed costs impacts individual analysis cost, one considered that, at the nuclear medicine center, approximately 140 quality control tests are performed every month, thus the depreciation value per analysis is R$ 1.95.
The quality control of the generator eluate must also be considered. The control of radionuclide purity depends upon a lead shielding, whose value is presented on Table 1, and for which the monthly depreciation value was calculated to be R$ 31.67. Considering that the generator is eluted twice daily, with an average of 40 elutions per month, the cost of the equipment for this type of analysis is R$ 0.79.
In the calculation of labor costs, the salaries plus social security taxes, vacation, hazard pay were considered for two groups of professionals in the area of nuclear medicine: the radiology technician (T) whose total cost was calculated to be R$ 1,767.00 and the college graduated in pharmacy, biomedicine or biology professionals (S), whose cost was calculated to be R$ 3,600.00. Considering that the radiology technician works 96 hours per month and the others work 176 hours, the value of the worked hour calculated for each category was R$ 18.40 and R$ 20.25 respectively. Also, it was considered that, as in practice several
kits are simultaneously labeled, the professionals will spend one hour of work to perform six quality controls, including the controls of the generator eluate, chromatographies and solvent extractions. Thus the cost of labor per analysis was calculated to be R$ 3.06 for the technologist and R$ 3.40 for the college graduated professional.
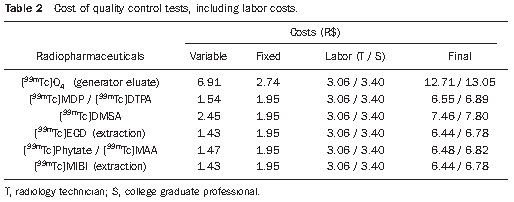
Table 2 presents the final cost of quality control of radiopharmaceuticals and [
99mTc] technetium eluates, based on the cost of consumables, fixed materials and labor.
DISCUSSION
It was considered that, for the deployment of a quality control program, the nuclear medicine clinic should purchase all the necessary materials for such program. The initial investment achieves approximately R$ 33,000.00 for permanent materials, with the largest part of such investment being related to the acquisition of the gamma counter (well counter) corresponding to 85% of the total investment, followed by the lead shielding required for the determination of (
99Mo) present in the generator eluate, corresponding to 10% of the total investment. Although the initial investment is apparently high, such value represents approximately 9% of the value of a tomographic scintillation camera with one detector, and the monthly depreciation value of the permanent materials of R$ 273.01 is roughly equivalent to the value received for a single bone scintigraphy study (R$ 190.99 – SUS reference value or R$ 259.12 AMB reference value).
The value of bone scintigraphy was taken as an example, as it is one of the most frequent studies performed in nuclear medicine clinics. Another very frequent study is [
99mTc] MIBI myocardial scintigraphy, at a cost of R$ 791.59 (SUS) or R$ 955.61 (AMB). In such a case, the value of the monthly depreciation of equipment corresponds to 1/3 of the value of a single perfusion myocardial scintigraphy.
In the cost assessment, permanent materials were considered as a fixed cost, i.e., the cost of the analysis is related to the rate of utilization of equipment and utensils. The calculated values between R$ 1.43 and R$ 2.54, may vary up or down according to the number of examinations performed in the center.
In the analysis of cost of control by solvent extraction, whose measurements are performed by means of a dose calibrator, the value involved in the acquisition of such equipment was not considered, since this is a mandatory item in the installation of nuclear medicine centers for labeling measurement and radiopharmaceuticals injection.
In the other item compounding the final cost, the initial investment for the acquisition of consumables achieves R$ 2,500.00, and such value is enough for the acquisition of materials in quantities for use along up to two years. With the recent Anvisa resolutions (14,15) and the obligation to perform quality control of all radiopharmaceuticals, it is possible that private suppliers start producing and marketing systems for quality control in quantities enough for a single month use, thus reducing the initial expenditure with consumables.
The selection of materials for each control was based on a compilation of procedures described in official compendiums, as determined by Anvisa, or in published scientific articles comparing different control procedures.
No significant difference was observed in values for the two methods utilized for analyses, chromatography and extraction by solvent (Table 2). As the extraction control is much faster (approximately 3 minutes) than control by chromatography (approximately 15 minutes), the first one was utilized for the quality control of [
99mTc] MIBI and [
99mTc] ECD radiopharmaceuticals, that are lipophilic, a necessary condition for the use of such process. However it should be highlighted that such method is not among the safest ones, due to the possibility of breakage of the analysis tubes and solvent spillage during manipulation.
The chromatographic method is mandatorily utilized in the evaluation of quality of other [
99mTc]technetium labeled radiopharmaceuticals. In such case, there are several possibilities of combination between the stationary and mobile phases. The systems described in the American(4) and European(5) pharmacopoeias are many times impracticable in the process performed in clinics for being expensive, time consuming and for requiring mixtures of solvents that are not usual in the daily practice of nuclear medicine centers. In practice, one utilizes the systems recommended by the radiopharmaceutical manufacturers or those recognized in the scientific literature describing particularly the utilization of Whatman 3MM paper or ITLC-SG, as stationary phase, and physiological solution and ketones (acetone or methyl ethyl ketone), as mobile phases.
Although ITLC-SG and Whatman 3MM paper may be indistinctively utilized in most analyses, the first one is better than the second one (better resolution in the separation of components and swiftness of the analysis – approximately 3 minutes). However, such advantages become of lesser importance when one compares the unit cost of R$ 1.36 for ITLC-SG and R$ 0.06 for Whatman 3MM paper. Thus, in the present study the utilization of Whatman 3MM paper was considered whenever such utilization was possible, of course with the exception for the analysis of [
99mTc] DMSA, for which TLC-SG is obligatorily necessary, at a cost of R$ 1.36.
As regards the time required for the analysis, the most significant limiting factor is the use of Whatman 3MM paper/saline solution, that takes approximately 12 minutes, while the system Whatman 3MM/acetone takes 5 minutes. When the analysis time is of primary concern at the institution, being such time as important as or more important than the cost, it is possible to utilize a mixed system of ITLC-SG/saline solution and Whatman 3MM/acetone.
Labor costs play a significant role, representing 40% to 50% of the total cost of the analyses. In the present study, within the utilized model, the utilization of professionals with different graduation levels and under different workday regimen is of little difference in the final cost of analysis (Table 2), provided the minimum base salary is considered for both categories, and that the professionals dedicate the established time for the performance of quality control. Variations in costs will depend upon regional or category collective labor agreements.
Finally, by analyzing Table 2, it is possible to observe that costs to perform quality control of [
99mTc] technetium labeled radiopharmaceuticals are relatively low, even considering the most extreme cost conditions, ranging from R$ 6.44 to R$ 7.80, depending upon the product or method to be adopted. The exception occurs for the control of the generator eluate, whose value achieves R$13.05, because of the higher number of analyses required to ensure the eluate quality.
In practice, the effective cost may be reduced, considering the fact that a labeled
kit is utilized in more than one patient.
CONCLUSIONS
In order to comply with the Anvisa resolutions RDC No. 38/2008 and RDC No. 63/2009 regarding the deployment of quality control programs for radiopharmaceuticals utilized in nuclear medicine centers, the authors conclude that, although the investment in equipment is relatively high, it actually represents a small fraction of the cost for set up and operation of a nuclear medicine center, and should therefore be automatically considered in the installation of new centers.
The final cost for quality control of [
99mTc]technetium labeled radiopharmaceuticals, between R$ 6.44 and R$ 7.80, can be absorbed in the cost of most nuclear medicine studies, particularly because the consumable contained in a single flask can be used for several patients, and the respective cost can be divided by that number of patients. Additionally, it is possible that the optimization of the work of the professional involved in the task, and the multiple utilization of the equipment in other functions in the clinic, may actually make the effective cost become sufficiently low so as not to affect the financial results of the nuclear medicine center.
REFERENCES
1. National Electrical Manufacturers Association. Performance measurements of scintillation cameras. NEMA Standards Publication NU 1-2001. Washington, DC: National Electrical Manufacturers Association; 2001.
2. International Atomic Energy Agency. Quality assurance for SPECT systems. IAEA Human Health Series No. 6. Vienna, Austria: International Atomic Energy Agency; 2009.
3. International Atomic Energy Agency. Quality control of nuclear medicine instruments. IAEA-TECDOC-602. Vienna, Austria: International Atomic Energy Agency; 1991.
4. United States Pharmacopeia. USP 33-NF-28. Rockville, MD: The United States Pharmacopeial Convention; 2010.
5. European Pharmacopoeia – 6th edition. Strasbourg, France: Council of Europe; 2007.
6. Comité de Radiofarmacia ALASBIMN, Mitta AEA, Robles AM. Manual de control de calidad de radiofármacos. Montevideo, Uruguay: Asociación Latinoamericana de Sociedades de Biología y Medicina Nuclear (ALASBIMN); 1986.
7. Brasil. Ministério da Ciência e Tecnologia. Comissão Nacional de Energia Nuclear. CNEN-NN-3.05: Requisitos de radioproteção e segurança para serviços de medicina nuclear. Rio de Janeiro, RJ: Comissão Nacional de Energia Nuclear; 1996.
8. Brasil. Ministério da Ciência e Tecnologia. Comissão Nacional de Energia Nuclear. CNEN-NE-3.01: Diretrizes básicas de radioproteção. Rio de Janeiro, RJ: Comissão Nacional de Energia Nuclear; 1988.
9. Brasil. Ministério da Ciência e Tecnologia. Comissão Nacional de Energia Nuclear. CNEN-NN-6.01: Requisitos para o registro de pessoas físicas para o preparo, uso e manuseio de fontes radioativas. Rio de Janeiro, RJ: Comissão Nacional de Energia Nuclear; 1998.
10. Marques FLN, Okamoto MRY, Buchpiguel CA. Alguns aspectos sobre geradores e radiofármacos de tecnécio-99m e seus controles de qualidade. Radiol Bras. 2001;34:233–9.
11. Ponto JA, Ponto LLB. Cost-effectiveness of routine radiochemical quality assurance testing of technetium Tc 99m radiopharmaceuticals. Am J Hosp Pharm. 1986;43:1218–22.
12. Decristoforo C, Chen F, Riccabona G. Qualitätskontrolle von Radiopharmaka in der Klinik – eine Notwendigkeit? Nuklearmedizin. 1993;32:144–8.
13. Faria DP, Marques FLN, Okamoto MRY, et al. Quality control of 99Mo/99mTc generator in two nuclear medicine laboratories in Brazil. In: V Scientific Meeting of the Brazilian Society of Nuclear Biosciences/I Encuentro Cientifico Hispanobrasileño; 2005 Ago 26–29; Belo Horizonte, MG, Brasil. Belo Horizonte: Anais do Evento (CD ROM).
14. Brasil. Ministério da Saúde. Agência Nacional de Vigilância Sanitária. Resolução no 38, de 4 de junho de 2008. Dispõe sobre a instalação e funcionamento de serviços de medicina nuclear “in vivo”. Brasília, DF: Diário Oficial da União, 18 de dezembro de 2008. Sec. 1, p. 175.
15. Brasil. Ministério da Saúde. Agência Nacional de Vigilância Sanitária. Resolução no 63, de 18 de dezembro de 2009. Dispõe sobre as boas práticas de fabricação de radiofármacos. Brasília, DF: Diário Oficial da União, 23 de dezembro de 2009. Sec. 1, p. 73.
16. Proulx A, Ballinger JR, Gulenchyn KY. Routine determination of radiochemical purity of 99mTc-MIBI. Int J Rad Appl Instrum A. 1989;40:95–7.
1. Pharmacist, Farmácia Escola da Universidade Positivo, Curitiba, PR, Brazil.
2. PhD, Chemist at Department of Radiology of Faculdade de Medicina da Universidade de São Paulo (FMUSP), São Paulo, SP, Brazil.
3. MD, Specialist in Nuclear Medicine, Director for Cermen – Medicina Nuclear, Curitiba, PR, Brazil.
4. PhD, Professor at Universidade Tecnológica Federal do Paraná (UTFPR), Curitiba, PR, Brazil.
Mailing Address:
Daniele de Paula Faria
Edifício Centro de Medicina Nuclear, Laboratório de Radiofarmácia
Rua Doutor Ovídio Pires de Campos, s/nº, Cerqueira César
São Paulo, SP, Brazil, 05403-010
E-mail: danielefaria1@gmail.com
Received June 11, 2010.
Accepted after revision October 29, 2010.
 Vol. 44 nº 1 - Jan. /Feb. of 2011
Vol. 44 nº 1 - Jan. /Feb. of 2011