INTRODUCTION
Lymphoma is the third most common malignant neoplasia in the childhood, after leukemia and central nervous system tumors. According to the mean gross rates observed in the records of cancer in the Brazilian population, the number of new cases of Hodgkin’s lymphoma in Brazil, in 2009, achieved approximately 1,600 in men and 1,270 in women(1).
The evaluation of the disease extent (staging) is important for an appropriate treatment planning and for determining the prognosis. Imaging methods play an essential role in the staging of lymphomas. Additionally, images are of great relevance in the monitoring of the response to therapy and in the detection of tumor recurrence(2–6).
Whole-body imaging in children is generally performed by scanning the entire skeleton, scintigraphy and positron emission tomography (PET), this latter being combined with computed tomography (CT). Exposure to ionizing radiation is a common characteristic in all these imaging methods, posing a negative effect that is more significant to children than to adults. Thus, alternative methods free of ionizing radiations are extremely important in pediatric radiology(7).
Over the past few years, there has been an increase in the application of whole-body magnetic resonance imaging (MRI) in adults, particularly in oncologic radiology. Such increase has been favored by the combination of fast sequences with the examination table motion and use of a body coil, or a specially designed platform with multiple coils.
In pediatrics, the main indication of such method has been the detection of bone marrow lesions(5,8), but the potential to extend this application to other systemic diseases is increasing.
Diffusion-weighted MRI provides functional data that may be utilized in the detection and characterization of pathological processes, malignant tumors inclusive(9–12). Therefore, such method may be valuable in the staging and follow-up of malignant tumors. In spite of the above mentioned developments in diffusion-weighted imaging, apnea was previously considered as necessary because respiratory motion impaired diffusion imaging(13–17). In 2004, Takahara et al.(18) described the concept of diffusion-weighted whole-body imaging with background body signal suppression (DWIBS). Such technique makes intentional use of free breathing instead of apnea, for the visualization of visceral organs and their lesions. In a later study published by Ballon et al.(19), DWIBS was also described with free breathing. Such study approached the visualization of metastatic lesions in static tissues (bone marrow), which was in agreement with the theory accepted at that time. However, those authors observed that visceral organs such as the spleen and the kidneys could also be analyzed.
Considering the hypothesis that differences in histologic cytoarchitecture is better reflected by the difference of free passage of water molecules, diffusion-weighted MRI must be the most sensitive method in the staging of metastases, with the advantage of being faster and capable of evaluating larger volumes than the other sequences(12).
A recent study reported that diffusion-weighted MRI is highly sensitive in the detection of malignant diseases. For that reason, diffusion-weighted whole-body imaging has been proposed as a powerful screening tool(11).
MATERIALS AND METHODS
In the period between March and November/2009, the authors developed a prospective study approaching the staging of Hodgkin’s lymphoma in 12 outpatients by means of whole-body MRI. The protocol was approved by the Committee for Ethics in Research of the institution, and a term of free and informed consent was signed by patients and their caregivers, after a verbal explanation on the document and the procedure. The results obtained in the present study did not affect the clinical approach to the patients included in the sample.
Patients with confirmed diagnosis of early-stage Hodgkin’s lymphoma or with clinical suspicion of recurrence were included. Patients whose diagnosis was not confirmed or those who did not present appropriate clinical conditions to undergo the examination were excluded.
The sample included seven male and five female patients aged between 12 and 24 years (mean age of 17.75 years). Among the 12 patients, six represented new cases and 6 presented suspicion of disease recurrence.
The studies were performed in an Achieva MRI equipment (Philips Medical Systems; Cleveland, OH, USA), with a body coil for signals transmission and reception. The patients were positioned in dorsal decubitus with the arms parallel to the body and the sequences were acquired with free-breathing. The utilization of intravenous paramagnetic contrast injection was not necessary and none of the patients required sedation. Based on the sagittal and coronal images, the acquisition volume was planned in four sections covering the entire body, with T1-weighted sequences (repetition time [TR] = 465 ms; echo time [TE] = 17 ms; field-of-view [FOV] = 515 mm; matrix = 512 × 512); T2-weighted sequences (TR = 1842 ms; TE = 80 ms; FOV = 515 mm; matrix = 512 × 512); STIR sequences (TR = 5420 ms; TE = 66 ms; FOV = 515 mm; matrix = 512 × 512) and DWIBS (TR = 6348 ms; TE = 70 ms; FOV = 515 mm; matrix = 336 × 336). Coronal T1-weighted, T2-weighted and STIR sequences were acquired with 7.0 mm slice thickness, and the DWIBS sequence was acquires in the cross-sectional, with 5.0 mm thick slices. The T1-weighted, T2-weighted and STIR sequences were reconstructed by means of the Mobiview technique, obtaining the fusion of the sections in the coronal plane, and the sequence DWIBS was reconstructed by means of the MIP and Mobiview techniques, also obtaining the fusion of sections in the coronal plane.
The interpretation of the images was made on workstations by two independent observers, both of them with experience in MRI and pediatric radiology. The images were randomly distributed, with the analysis of sequences of a same patient being performed in different days. Sites of possible involvement by lymphoma were established, four of such locations being nodal (neck, chest, abdomen and pelvis) and eight extranodal (lung, chest wall, liver, spleen, pancreas, intestinal loops and bone marrow). The sites were classified as positive in cases where the lymph nodes were > 1.0 cm on their smallest axes or in the presence of signal alterations (hypo/isosignal on T1-weighted and T2-weighted sequences and hypersignal intensity on STIR or DWIBS sequences) and/or masses.
The number and localization of involved sites as well as the staging of each one of them were compared among the different sequences.
The following statistical analyses were made:
– interobserver agreement on results in relation to lymph node chains;
– interobserver agreement on results in relation to parenchymal organs;
– interobserver agreement on results in relation to bone marrow;
– interobserver agreement on results of the four sequences, to find which one identifies the highest number of lesions resulting from the lymphoma.
RESULTS
All the images were technically appropriate, and no technique-related complication was observed. All the images presented a good quality, despite the presence of artifacts which did not negatively affect the diagnosis. Such artifacts occurred predominantly in the chest and abdomen, and were caused by breathing, heartbeats and/or body motion (Figure 1).
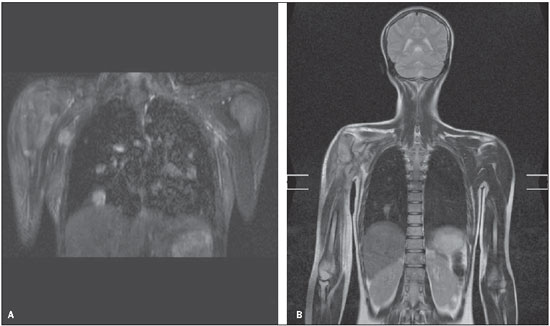
Figure 1. Heartbeat (A) and respiratory artifacts (B), that did not impair the detection of pulmonary nodules.
The fastest sequences were the T1- and T2-weighted ones, taking four minutes and 16 seconds to be acquired. The STIR sequences took longer, seven minutes and four seconds. The DWIBS took the longest time to be acquires, 12 minutes and 52 seconds.
The time required by each observer to evaluate the images was measured. The longest a time was observed in the analysis of the DWIBS sequences, with a mean time of 133 seconds for the observer 1 and 140 seconds for the observer 2. The other sequences presented similar results, with a mean required time ranging from 109 to 116 seconds.
The number of lymph node sites characterized as affected by the disease on T1- and T2-weighted sequences was similar (8 sites for both sequences), however it was lower than the numbers observed on STIR and DWIBS sequences (11 and 12 sites, respectively). The difference between these methods was due to an axillary lymph node interpreted as affected by the disease when visualized at the DWIBS sequence, and that was not identified at the STIR sequence (Figure 2), requiring further evaluation by ultrasonography, which identified an axillary lymph node with increased dimensions, associated with loss of habitual morphology and hyper vascular flow at Doppler.
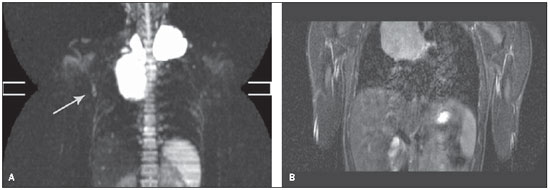
Figure 2. A: Axillary lymph node at right (arrow) visualized on the DWIBS sequence, which were not identified on the STIR sequence (B).
In the analysis of involvement of parenchymal organs, all the sequences presented similar results. As regards bone marrow involvement by lymphoma, the same number of lesions (17) was observed on T1-, T2-weighted, and DWIBS sequences, superior to the number of lesions (13) observed on STIR sequences.
In some cases, hypersignal intensity was identified on the DWIBS sequence, classified as non representative. In one patient, hypersignal intensity secondary to biopsy was observed on the right iliac crest (Figure 3). In two patients, moderate and diffuse hypersignal intensity was visualized in the bone marrow (Figure 4). In all the cases the spleen presented hypersignal intensity (Figure 4), a typical finding due to the organ vascularization(18,20).
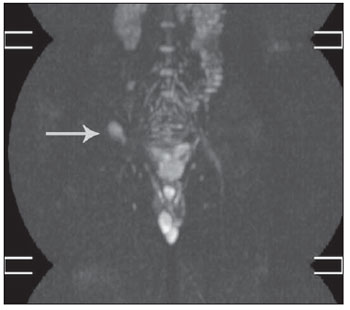
Figure 3. Hypersignal intensity secondary to biopsy at the right iliac crest (arrow).
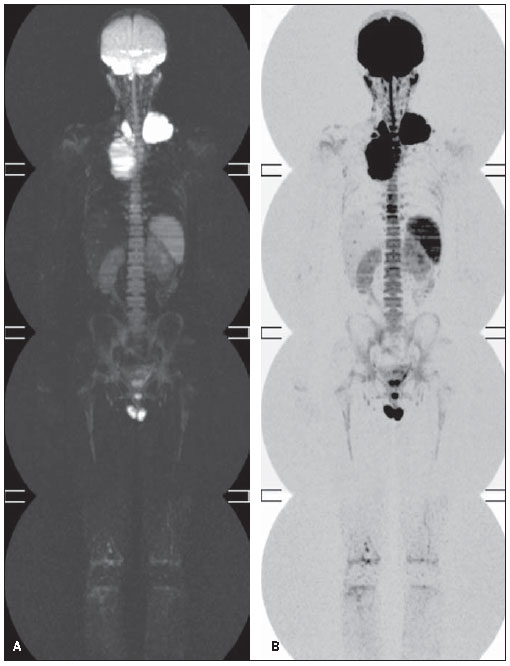
Figure 4. A: Diffuse hypersignal intensity in the bone marrow. B: black and white scale inversion.
After the analysis of the images, the patients were staged at each sequence, according to the Ann Arbor criteria. No differences were detected in the staging performed by the two observers, but divergence was observed in two cases as regards staging, i.e., higher stages (stages II and III) were observed on STIR and DWIBS sequences as compared with the T1- and T2-weighets sequences (stage I).
The kappa test was utilized in the analysis of the interobserver agreement, considering a significance level corresponding to 5%. In the comparison of the results obtained by the two observers in the interpretation of the STIR images, a very high degree of interobserver agreement was observed (kappa index ranging from 0.816 to 1). Similar results were observed in the analysis of the T2-weighted sequences, with the kappa index ranging from 0.80 to 1. As regards the DWIBS sequence, interobserver agreement was observed (p < 0.05), although not at the same level as the previous methods (kappa index ranging from 0.571 to 1). The interobserver agreement was similar on the T1-weighted and DWIBS sequences (kappa index ranging from 0.625 to 1).
DISCUSSION
There are various potential advantages of whole-body MRI as compared with conventional imaging methods in the staging of Hodgkin’s lymphoma. Considering that the exposure to ionizing radiation, even at small doses, may increase the risk for secondary neoplasias in children(21–23), whole-body MRI, by not relying on ionizing radiation, might be utilized as an alternative imaging method in the staging of lymphomas. Additionally, there is no need for either oral or intravenous administration of contrast agents, and the evaluation of the disease extent seems to be feasible with the utilization of a single imaging method, resulting in costs reduction, besides reduction in the need for sedation and in the number of visits of the patient to the imaging center.
The whole-body MRI feasibility has been demonstrated in a number of diseases(24–28). Some studies with adult patients with lymphoma have evaluated the utilization of MRI, with CT as a reference standard. By using such comparison, Brennan et al.(29) have reported that the STIR sequence could be used with accuracy in the identification of lymph nodes > 1.2 cm.
In the follow-up and staging of lymphomas, whole-body MRI allows the detection of involvement of bone marrow, lymph nodes and parenchymal organs by this disease. Because of the potentiality of the method, the authors decided to compare four whole-body MRI sequences (T1-weighted, T2-weightes, STIR and DWIBS), evaluating the time required for the images acquisition, images quality, identification of the lesions, interobserver agreement and mean analysis time for each sequence.
The shorter time for interpretation of T1-weighted, T2-weighted and STIR images was attributed to the greater spatial resolution of such sequences. The STIR sequence presented higher interobserver agreement because of the greater contrast resolution between the lesions and the adjacent healthy tissue.
The two patients who presented diffuse moderate hypersignal intensity on the bone marrow were utilizing Granulokyne
® at the time of the images acquisition. Maybe, the utilization of such medication have been the cause for the hypersignal intensity, as in the follow-up no sign of involvement of such regions by lymphoma was observed.
Although studies in the literature report the higher sensitivity of diffusion-weighted MRI in the staging of metastases, as well as in the monitoring of the response to chemotherapy in some cases, significant differences were not observed in the present study with respect to the STIR sequence.
The present study faced some limitations, such as the small size of the sample and the impossibility of histological confirmation of the abnormalities characterized at MRI.
At MRI, alteration of the signal intensity and/or lesion size is utilized as diagnostic criterion for the presence of tumor activity. On the other hand, PET-CT evaluates the tumor metabolism based on the increase in the glycolytic activity. As both diagnostic methods are utilized in the staging and follow-up of oncologic patients, the continuation of the present study shall be undertaken with the correlation of whole-body MRI with PET-CT images.
CONCLUSIONS
In the analysis of lymph node sites characterized as affected by the disease, the STIR and DWIBS sequences detected a higher number of lesions. All the sequences presented similar results in the evaluation of parenchymal organs and bone marrow. The interobserver agreement was high for all the analyzed sequences, with the best results being observed at the STIR sequence.
REFERENCES
1. Brasil. Ministério da Saúde. Instituto Nacional de Câncer. Estimativa 2010: incidência de câncer no Brasil. [acessado em 20 de maio de 2010]. Disponível em: http://www.inca.gov.br/estimativa/2010/
2. Carty H, Martin J. Staging of lymphoma in childhood. Clin Radiol. 1993;48:151–9.
3. Halliday T, Baxter G. Lymphoma: pictorial review. II. Eur Radiol. 2003;13:1224–34.
4. Halliday T, Baxter G. Lymphoma: pictorial review. I. Eur Radiol. 2003;13:1154–64.
5. Hamrick-Turner JE, Saif MF, Powers CI, et al. Imaging of childhood non-Hodgkin lymphoma: assessment by histologic subtype. Radiographics. 1994;14:11–28.
6. Weinstein HJ, Tarbell NJ. Leukemias and lymphomas of childhood. In: DeVita VT, Hellman S, Rosenberg SA, editors. Cancer: principles and practice of oncology. Philadelphia: Lippincott Williams & Wilkins; 2001. p. 2235–56.
7. Kellenberger CJ, Miller SF, Khan M, et al. Initial experience with FSE STIR whole-body MR imaging for staging lymphoma in children. Eur Radiol. 2004;14:1829–41.
8. Rosenthal H, Kolb R, Gratz KF, et al. Bone manifestations in non-Hodgkin’s lymphoma in childhood and adolescence. Radiologe. 2000;40:737–44.
9. Golder WA. Lymph node diagnosis in oncologic imaging: a dilemma still waiting to be solved. Onkologie. 2004;27:194–9.
10. Pieterman RM, van Putten JW, Meuzelaar JJ, et al. Preoperative staging of non-small-cell lung cancer with positron-emission tomography. N Engl J Med. 2000;343:254–61.
11. Schmidt GP, Reiser MF, Baur-Melnyk A. Whole-body MRI for the staging and follow-up of patients with metastasis. Eur J Radiol. 2009;70:393–400.
12. Kwee TC, Takahara T, Ochiai R, et al. Diffusion-weighted whole-body imaging with background body signal suppression (DWIBS): features and potential applications in oncology. Eur Radiol. 2008;18:1937–52.
13. Low RN, Gurney J. Diffusion-weighted MRI (DWI) in the oncology patient: value of breathhold DWI compared to unenhanced and gadolinium-enhanced MRI. J Magn Reson Imaging. 2007;25:848–58.
14. Nasu K, Kuroki Y, Kuroki S, et al. Diffusion-weighted single shot echo planar imaging of colorectal cancer using a sensitivity-encoding technique. Jpn J Clin Oncol. 2004;34:620–6.
15. Nasu K, Kuroki Y, Nawano S, et al. Hepatic metastases: diffusion-weighted sensitivity-encoding versus SPIO-enhanced MR imaging. Radiology. 2006;239:122–30.
16. Taouli B, Martin AJ, Qayyum A, et al. Parallel imaging and diffusion tensor imaging for diffusion-weighted MRI of the liver: preliminary experience in healthy volunteers. AJR Am J Roentgenol. 2004;183:677–80.
17. Yoshikawa T, Kawamitsu H, Mitchell DG, et al. ADC measurement of abdominal organs and lesions using parallel imaging technique. AJR Am J Roentgenol. 2006;187:1521–30.
18. Takahara T, Imai Y, Yamashita T, et al. Diffusion weighted whole body imaging with background body signal suppression (DWIBS): technical improvement using free breathing, STIR and high resolution 3D display. Radiat Med. 2004;22:275–82.
19. Ballon D, Watts R, Dyke JP, et al. Imaging therapeutic response in human bone marrow using rapid whole-body MRI. Magn Reson Med. 2004;52:1234–8.
20. Koh DM, Collins DJ. Diffusion-weighted MRI in the body: applications and challenges in oncology. AJR Am J Roentgenol. 2007;188:1622–35.
21. Pierce DA, Preston DL. Radiation-related cancer risks at low doses among atomic bomb survivors. Radiat Res. 2000;154:178–86.
22. Brenner D, Elliston C, Hall E, et al. Estimated risks of radiation-induced fatal cancer from pediatric CT. AJR Am J Roentgenol. 2001;176:289–96.
23. Kleinerman RA. Cancer risks following diagnostic and therapeutic radiation exposure in children. Pediatr Radiol. 2006;36 Suppl 2:121–5.
24. Daldrup-Link HE, Franzius C, Link TM, et al. Whole-body MR imaging for detection of bone metastases in children and young adults: comparison with skeletal scintigraphy and FDG PET. AJR Am J Roentgenol. 2001;177:229–36.
25. Goo HW, Choi SH, Ghim T, et al. Whole-body MRI of paediatric malignant tumours: comparison with conventional oncological imaging methods. Pediatr Radiol. 2005;35:766–73.
26. Kellenberger CJ, Epelman M, Miller SF, et al. Fast STIR whole-body MR imaging in children. Radiographics. 2004;24:1317–30.
27. Kumar J, Seith A, Kumar A, et al. Whole-body MR imaging with the use of parallel imaging for detection of skeletal metastases in pediatric patients with small-cell neoplasms: comparison with skeletal scintigraphy and FDG PET/CT. Pediatr Radiol. 2008;38:953–62.
28. Laffan EE, O’Connor R, Ryan SP, et al. Whole-body magnetic resonance imaging: a useful additional sequence in paediatric imaging. Pediatr Radiol. 2004;34:472–80.
29. Brennan DD, Gleeson T, Coate LE, et al. A comparison of whole-body MRI and CT for the staging of lymphoma. AJR Am J Roentgenol. 2005;185:711–6.
1. MD, Radiologist, Fellow Master degree, Departamento de Diagnóstico por Imagem da Universidade Federal de São Paulo (Unifesp), São Paulo, SP, Brazil.
2. PhD, Associate Professor, Head of Division of Imaging Diagnosis in Pediatrics, Departamento de Diagnóstico por Imagem da Universidade Federal de São Paulo (Unifesp), São Paulo, SP, Brazil.
3. PhD, MD, Oncologist, Instituto de Oncologia Pediátrica (IOP/GRAACC) da Universidade Federal de São Paulo (Unifesp), São Paulo, SP, Brazil.
4. MD, Radiologist, Clínica Centrus, Campinas, SP, Brazil.
5. PhD, Titular Professor of Radiology, Department of Imaging Diagnosis, Head of the Center of Imaging Diagnosis at Instituto de Oncologia Pediátrica (IOP/GRAACC) da Universidade Federal de São Paulo (Unifesp), São Paulo, SP, Brazil.
Mailing Address:
Dr. Daniel Nava
Avenida Francisco Glicério, 2132, Vila Itapura
Campinas, SP, Brazil, 13023-100
E-mail: daniel@centrus.com.br
Received August 25, 2010.
Accepted after revision November 10, 2010.
* Study developed at Instituto de Oncologia Pediátrica (IOP/GRAACC) da Universidade Federal de São Paulo (Unifesp), São Paulo, SP, and at Lúmen – Centro de Diagnósticos, São Bernardo do Campo, SP, Brazil.
 Vol. 44 nº 1 - Jan. /Feb. of 2011
Vol. 44 nº 1 - Jan. /Feb. of 2011



