INTRODUCTION
The human immunodeficiency virus (HIV) is a retrovirus that penetrates the nervous system cells producing lesions(1) and impairing cognitive, motor and behavioral functions, many times resulting in dementia(2). The progress in the development of the highly active antiretroviral therapy (HAART) has allowed a significant reduction in the mortality of patients infected with HIV and, consequently, has aided them to achieve the aging phase of the lifecycle(3,4). According to the World Health Organization(5,6), the prevalence of cases of HIV/AIDS still remains quite high, affecting about 730 thousand individuals in 2008, just in Brazil. Such data indicate the greater longevity of these patients, but also demonstrate an increase in the incidence of neurocognitive complications frequently observed in the elderly populations. Such complications, in association with aging, become a significant problem for HIV-positive patients(3,7–9). Thus, the current reality enhances the need for a better clinical and imaging evaluation of patients with HIV/AIDS with possible cognitive deficit(10).
Advanced techniques of magnetic resonance imaging (MRI), including images acquisition and processing, have been utilized in the assessment of patients at advanced stages of cognitive deficit, particularly those who have progressed to a demential state(11). Such techniques seek to find out morphological or functional alterations in patients with no significant finding at conventional MRI. Studies approaching such imaging techniques have demonstrated a good correlation with neuropsychological tests (NPT)(11,12), clinically validating the eventual findings. Investigations approaching imaging methods have already been developed with some samples of HIV/AIDS patients(12,13). Thompson et al.(12) have found significant cortical atrophy in the gray matter of AIDS patients, involving the following areas: primary sensorimotor, premotor, frontopolar, language-related frontal and temporal lobes, pre-frontal and parietal areas, with significant correlation between these two latter areas and performance in neuropsychological tests. Complementarily, Chiang et al.(13) have observed severe atrophy in both, the primary and sensorimotor association areas of both hemispheres. Such alterations, particularly those in the white matter, observed on images, also present significant correlation with neuropsychological tests results. In such study, additionally to the mentioned findings, the authors have observed volumetric decrease in the frontal, medial and basal regions, in the middle segment of the cingulate gyrus and corpus callosum genu(13).
Also, the frontostriatal areas may be injured by the human immunodeficiency virus at early stages of the disease. Generally, lesions in such areas are associated with executive functions deficits(14,15). Moreover, the frontal cortex is closely related to the processing of executive functions, particularly the dorsolateral prefrontal system as well as the basal ganglia and the posterior parietal cortex(16). The literature has suggested that executive functions deficits has been one of the core neurocognitive impairments in HIV-positive patients(17). Such functions comprehend several cognitive processes which, as a whole, control and monitor other cerebral functions to achieve goal-oriented behaviors. These functions facilitate the planning and adaptation to new situations(18,19) and support appropriate functioning of other related cognitive domains, such as, memory.
Despite the possible damages caused by HIV to the executive functions, few studies are found in the literature investigating the correlation between cortical thickness in such regions and performance in neuropsychological tests that evaluate cognitive components of executive functions in HIV/AIDS patients(20,21).
Considering the relevance of this type of investigation, the present study was aimed to correlate the frontal cortical thickness with performance in neuropsychological tests that evaluate executive functions in HIV-positive patients. As a hypothesis, it was expected that HIV patients presented correlations between cortical thickness of frontal areas and their performances, particularly in tests evaluating inhibition.
MATERIALS AND METHODS
Study sample
The present study evaluated 22 HIV-positive patients (5 women and 17 men; mean age 52.91 years, standard deviation (SD) = 5.879; mean education level = 11.95 years, SD = 4.541). The patients were undergoing clinical follow-up at Hospital Universitário Clementino Fraga Filho (HUCFF) da Universidade Federal do Rio de Janeiro (UFRJ), Rio de Janeiro, Brazil. Inclusion criteria were the following: age range between 45 and 65 years, diagnosis of HIV infection for at least five years, absence of previous history of any type of neurological disease, currently undergoing treatment with HAART, absence of uncorrected auditory and/or visual alterations, mini-mental state examination score
> 17, for patients with schooling
< 4 years, or
> 24 for patients with schooling
> 5 years.
In compliance with ethical principles, all the individuals in this study participated on a voluntary basis, received no remuneration, and signed a term of free and informed consent. This study was approved by the Committee for Ethics in Research of HUCFF-UFRJ (CEP/UFRJ No. 151/08).
Data collection
The evaluation of these patients was conducted in two parts: MRI and neuropsychological assessment. The MRI studies were performed at HUCFF in a 1.5 tesla Avanto system (Siemens Medical Systems, Erlangen, Germany) with an eight-channel head coil. The following sequences were acquired: axial FLAIR [repetition time (TR): 9000 ms; echo time (TE): 83 ms; inversion time (TI): 2500 ms;
flip angle: 180°; matrix: 256 × 256;
field-of-view (FOV): 230 mm), 3D sagittal T1-weighted (TR: 2530 ms; TE: 3.39 ms; TI: 1100 ms;
flip angle: 7°;
voxel: 1.33 mm
3), coronal T2-weighted (TR: 3500 ms; TE: 99 ms;
flip angle: 136°; matrix: 256 × 256; FOV: 210 mm),diffusion tensor imaging (TR: 1900 ms; TE: 81 ms; matrix: 256 × 256; FOV: 230 mm; B = 0 s/mm
2 and B = 1000 s/mm
2, six gradient directions). All the conventional magnetic resonance images were evaluated by two experienced radiologists and were considered as normal, except for the presence of different degrees of cortical atrophy.
The MRI T1-weighted images for the study of cortical thickness were processed by means of the FreeSurfer v4.0.5 (Martinos Center, Boston, USA). Technical post-processing details were described in previous publications(22,23). In summary, the images processing included the following steps: motion correction, removal of non-cerebral tissue, automated Talairach transformation, intensity normalization, sub-cortical white matter and deep grey matter and deep grey matter segmentation, cortical reconstruction and subdivision, brain insufflation and cortical thickness mapping. Specifically for the present study the mean cortical thicknesses of the following regions in both hemispheres were evaluated: pre-frontal, lateral orbitofrontal, upper frontal and caudal level of anterior cingulum (Figure 1).
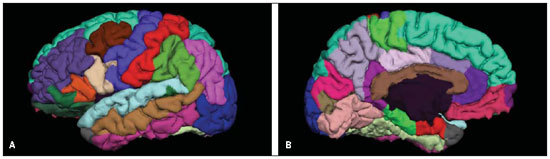
Figure 1. Lateral (a) and medial (b) views of left cerebral hemisphere, with segmentation of the different cerebral gyri with the aid of the FreeSurfer software.
The neuropsychological battery was administered by experienced and trained neuropsychologists. The evaluation was accomplished in a single session of approximately 90 minutes. Furthermore, the standardized instruments selected to measure cognitive components of the executive functions, were the ones most frequently found in the literature on HIV-positive patients(24), such as, processing speed, inhibition, verbal fluency (initiation and verbal planning), central executive component of working memory, cognitive flexibility, organization, selection and strategies maintenance, among others. The neuropsychological instruments administration sequence was planned in a way to minimize the effects of interference among them. Instruments with predominantly verbal tasks were alternated with non-verbal tasks, as follows:
1. Trail Making Test(25) – comprises two parts as follow: part (A) analyzes visual-motor coordination, processing speed and concentrated attention; part (B) besides the cognitive functions of part A, it also investigates alternate attention, cognitive flexibility and inhibition.
2. Wisconsin Card Sorting Test(26) – Evaluates organization, planning, categorization, inhibition, cognitive flexibility and rules learning.
3. Hayling test(27) – Evaluates verbal initiation (part A), verbal inhibition and processing speed (part B).
4. Digits subtest of the Wechsler Adult Intelligence Scale (WAIS-III)(28) – Analyses concentrated attention and short-term memory (direct order recall), besides the central executive component of working memory (indirect order recall).
5. Subtest of orthographic verbal fluency of Montreal Communication Assessment Battery (MAC Battery)(29) – investigates verbal initiation, inhibition, lexical memory and language.
6. Stroop Color and Word Test(30) – Verifies concentrated attention, inhibition, processing speed and cognitive flexibility.
7. Mini-Mental State Exam(31) – is a test for cognitive screening of suggestive signs of dementia, employed for characterizing the sample.
Statistical analysis
The data obtained by neuroimaging and neuropsychological assessments were correlated by the Pearson's correlation coefficient,
p < 0.05. The Statistical Package for the Social Sciences (SPSS) 16.0 was used for this analysis.
RESULTS
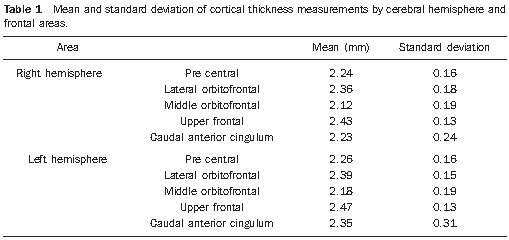
Mean values and standard deviation of the cortical thickness in the different regions evaluated are shown on Table 1.
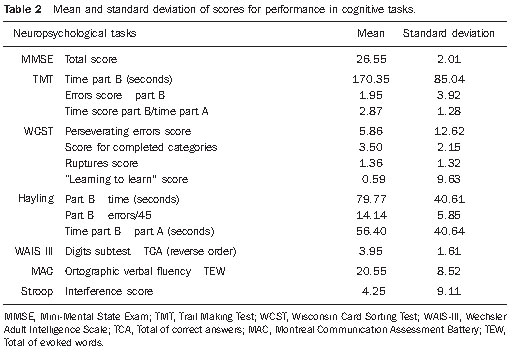
Table 2 shows mean scores and standard deviations of the neuropsychological performance observed in the executive functions tests.
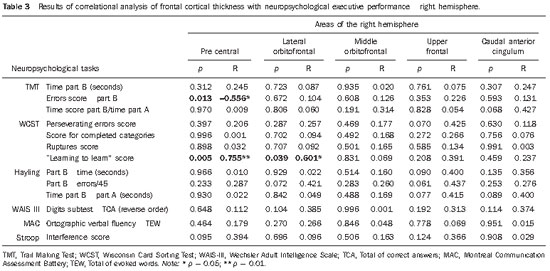
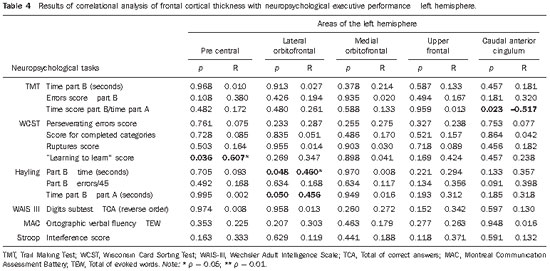
Results regarding the correlation between cortical thickness and performance on instruments for assessing executive functions are shown on Tables 3 (right hemisphere) and 4 (left hemisphere).
Tables 3 and 4 demonstrate moderately to strongly significant positive correlation between Wisconsin test scores and cortical thickness in the pre-central and lateral orbitofrontal regions at right, and left pre-central region. Also, a significant negative correlation was observed between the Trail Making Test scores and the cortical thickness in the right pre-central region and left anterior caudal cingulum. Finally, moderately significant correlation was observed between Hayling Test scores and cortical thickness in the left lateral orbitofrontal region.
DISCUSSION
The present study investigated the association between measures of cortical thickness of frontal and pre-frontal regions and performance scores on cognitive tasks that assessed executive functions of HIV-positive patients. The hypothesis of an association between morphological brain measurements at MRI and neuropsychological measurements was confirmed, and significant (both positive and negative) correlations were found.
With regard to correlations found in the Trail Making Test, the score that was negatively associated with cortical thickness of the left caudal anterior cingulate,was the time required to complete Part B of this task. Such index represents the measurement of the processing speed, connected with inhibition, cognitive flexibility and alternate attention. Thus, the longer the time required for HIV-positive patients to complete the part B of this test, the thinner was the cortical thickness of this region, suggesting that slowing down in the processing speed of these patients, may be related to the decrease in the thickness of the anterior third of the cingulate gyrus. Such region has been related to the executive functions, particularly the inhibition(32). As a complement, the score corresponding to the number of errors in the most complex part of the Trail Making Test was negatively correlated with the cortical thickness of the right pre-central region, indicating that the greater the amount of errors, the thinner is the cortical thickness of this area in the evaluated patients. Also, such region has frequently been associated with executive components, particularly in patients with personality disorders(33).
Correlations regarding the Wisconsin test reinforce the findings discussed above of the Trail Making Test, that the learning strategies scores were positively correlated with the cortical thickness of the pre-central region (bilateral). This may suggest that the HIV-positive patients' difficulty in learning with the previously employed strategy, as well as, their difficulty in solving problems (learning-to-learn score)(34) must be related to the decrease in the cortical thickness of this region. Additionally to this correlation, a positive association was observed between this score and the right lateral orbitofrontal thickness, a region that is related to executive components measured by the learning-to-learn score(35).
In what concerns the relationship of scores of time to complete Hayling test part B, they predominantly occurred with the left hemisphere, at the lateral orbitofrontal region. Such finding corroborates the one already mentioned on the Wisconsin test score. However, its interpretation implies the correlation between longer time required to complete this complex part of the cognitive task and greater cortical thickness in the evaluated areas. Such data regarding Hayling test scores were not expected, neither had previously been described, possibly indicating an increase in the cortical thickness in the mentioned regions, allowing the HIV-positive patients to demonstrate a better performance and a possible neuronal plasticity.
Negative correlations observed between performance in the Trail Making test part B and pre-frontal areas of the right hemisphere and caudal anterior cingulum, in the left hemisphere, are similar to some findings reported in the literature on the correlation between decrease in the cortical thickness and worsening of the processing speed(21,21). As regards the Stroop Color and Word test evaluating concentrated attention, inhibition, processing speed and cognitive flexibility, the present study did not observed any correlation between cortical thickness, particularly in the frontal cortex, and executive functions. However, this type of correlation was observed in a study with HIV-positive patients, but without using antiretroviral therapy(36).
Overall, the present study may be considered as relatively pioneering at national level, with tangential investigations in the international literature. However, the present findings have a preliminary, exploratory character. Thus, one must consider the limitations represented by the small size of the sample and the correlational delineation that did not include a control group of healthy individuals or a comparative group of HIV-positive patients with no antiretroviral treatment.
Another implication is the cross-sectional design of the study, which restricts comparisons of the cognitive deficits progression over time and also restricts a wealthy source of information of comparing the progression of HIV related cognitive deficits with the patient's baseline.
Longitudinal investigations are required to evaluate how antiretroviral therapies affect white substance lesions in HIV-positive patients(37), and also can more clearly demonstrate the association between frontal cortex atrophy and the executive functioning. Finally, another limitation involved the correlation between many cognitive and neuroanatomical variables which, for the purpose of the present preliminary study, demonstrate promising relationships that should be more deeply investigated and specified in future studies. Considering that executive functions are critical in the daily life, further studies in this area may guide practitioners not only in their clinical practice, but also in the research of effective therapies to reduce cognitive deficits in HIV-positive individuals. Such interventional actions with both, medications and at cognitive level, may contribute to prevent or minimize executive functions impairments before the progression to dementia associated with HIV infection.
Finally, based on the results of the present study with 22 HIV-positive patients, a correlation can be observed between frontal cortical thickness and performance in neuropsychological executive functions tests. The correlations demonstrated in the present study suggest that executive deficits in HIV-positive patients are related to a decrease in the cortical thickness of several frontal regions, most significantly in the left hemisphere. Positive correlations indicating increase in the thickness at some regions may be related to the neuronal plasticity in HIV patients(38). Further studies are required to corroborate such results, demonstrating more clearly evidences of the interface between neuroradiology and neuropsychology in the setting of neurodegenerative disorders related to HIV infection.
Acknowledgements
To the following research funding institutions: Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (Capes), Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) and Fundação de Amparo à Pesquisa do Estado do Rio de Janeiro (Faperj).
REFERENCES
1. Christo PP. Alterações cognitivas na infecção pelo HIV e AIDS. Rev Assoc Med Bras. 2010;56:242–7.
2. Bottiggi KA, Chang JJ, Schmitt FA, et al. The HIV dementia scale: predictive power in mild dementia and HAART. J Neurol Sci. 2007;260:11–5.
3. Cysique LA, Brew BJ. Neuropsychological functioning and antiretroviral treatment in HIV/AIDS: a review. Neuropsychol Rev. 2009;19:169–85.
4. Woods SP, Moore DJ, Weber E, et al. Cognitive neuropsychology of HIV-associated cognitive disorders. Neuropsychol Rev. 2009;19:152–68.
5. World Health Organization. Global summary of the HIV/AIDS epidemic, December 2008. [acessado em 26 de outubro de 2010]. Disponível em: http://www.who.int/hiv/data/2009_global_summary.gif
6. World Health Organization. Epidemological fact sheet on HIV and AIDS: core data on epidemiology and response – Brazil, December 2008. [acessado em 26 de outubro de 2010]. Disponível em: http://www.who.int/countries/bra/en/
7. Tozzi V, Balestra P, Bellagamba R, et al. Persistence of neuropsychologic deficits despite long-term highly active antiretroviral therapy in patients with HIV-related neurocognitive impairment: prevalence and risk factors. J Acquir Immune Defic Syndr. 2007;45:174–82.
8. Deeks SG. HIV infection, inflammation, immunosenescence, and aging. Annu Rev Med. 2011;62:141–55.
9. Brew BJ, Crowe SM, Landay A, et al. Neurodegeneration and ageing in the HAART era. J Neuroimmune Pharmacol. 2009;4:163–74.
10. Kochunov P, Robin DA, Royall DR, et al. Can structural MRI indices of cerebral integrity track cognitive trends in executive control function during normal maturation and adulthood? Hum Brain Mapp. 2009;30:2581–94.
11. Sjöbeck M, Elfgren C, Larsson EM, et al. Alzheimer's disease (AD) and executive dysfunction. A case-control study on the significance of frontal white matter changes detected by diffusion tensor imaging (DTI). Arch Gerontol Geriatr. 2010;50:260–6.
12. Thompson PM, Dutton RA, Hayashi KM, et al. Thinning of the cerebral cortex visualized in HIV/AIDS reflects CD4+ T lymphocyte decline. Proc Natl Acad Sci U S A. 2005;102:15647–52.
13. Chiang MC, Dutton RA, Hayashi KM, et al. 3D pattern of brain atrophy in HIV/AIDS visualized using tensor-based morphometry. Neuroimage. 2007;34:44–60.
14. Gunning-Dixon FM, Murphy CF, Alexopoulos GS, et al. Executive dysfunction in elderly bipolar manic patients. Am J Geriatr Psychiatry. 2008;16:506–12.
15. Melrose RJ, Tinaz S, Castelo JMB, et al. Compromised fronto-striatal functioning in HIV: an fMRI investigation of semantic event sequencing. Behav Brain Res. 2008;188:337–47.
16. Stuss DT, Levine B. Adult clinical neuropsychology: lessons from studies of the frontal lobes. Annu Rev Psychol. 2002;53:401–33.
17. Dawes S, Suarez P, Casey CY, et al. Variable patterns of neuropsychological performance in HIV-1 infection. J Clin Exp Neuropsychol. 2008;30:613–26.
18. Collette F, Van der Linden M, Laureys S, et al. Exploring the unity and diversity of the neural substrates of executive functioning. Hum Brain Mapp. 2005;25:409–23.
19. Collette F, Hogge M, Salmon E, et al. Exploration of the neural substrates of executive functioning by functional neuroimaging. Neuroscience. 2006;139:209–21.
20. Harrison MJG, Newman SP, Hall-Craggs MA, et al. Evidence of CNS impairment in HIV infection: clinical, neuropsychological, EEG, and MRI/MRS study. J Neurol Neurosurg Psychiatry. 1998;65:301–7.
21. Poutiainen E, Elovaara I, Raininko R, et al. Cognitive performance in HIV-1 infection: relationship to severity of disease brain atrophy. Acta Neurol Scand. 1993;87:88–94.
22. Fischl B, Salat DH, Busa E, et al. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron. 2002;33:341–55.
23. Desikan RS, Sgonne F, Fischl B, et al. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage. 2006;31:968–80.
24. Rippeth JD, Heaton RK, Carey CL, et al. Methamphetamine dependence increases risk of neupsychological impairment in HIV infected persons. J Int Neuropsychol Soc. 2004;10:1–14.
25. Fonseca RP, Grassi-Oliveira R, Oliveira CR, et al. Instruments of executive functions assessment: preliminary normative data and sociodemographic studies. Dement Neuropsychol. In Press.
26. Cunha JA, Trentini CM, Argimon IL, et al. Teste Wisconsin de classificação de cartas – adaptação e padronização brasileira. São Paulo, SP: Casa do Psicólogo; 2005.
27. Fonseca RP, Oliveira CR, Gindri G, et al. Teste Hayling: um instrumento de avaliação de componentes das funções executivas. In: Hutz CS, organizador. Avanços em avaliação psicológica e neuropsicológica de crianças e adolescentes. 1ª ed. São Paulo, SP: Casa do Psicólogo; 2010. p. 337–64.
28. Nascimento E. Adaptação, validação e normatização do WAIS-III para uma amostra brasileira. In: Wechsler D. WAIS-III: manual para administração e avaliação. São Paulo, SP: Casa do Psicólogo; 2004. p. 161–92.
29. Fonseca RP, Parente MAMP, Cote H, et al. Bateria Montreal de avaliação da comunicação – Bateria MAC. Barueri, SP: Pró-Fono; 2008.
30. Strauss E, Sherman EMS, Spreen O. Executive functions. In: Strauss E, Sherman EMS, Spreen O, editors. A compendium of neuropsychological tests: administration, norms, and commentary. 3rd ed. New York, NY: Oxford University Press; 2006. p. 401–545.
31. Almeida OP. Mini exame do estado mental e o diagnóstico de demência no Brasil. Arq Neuropsiquiatr. 1998;56:605–12.
32. Hoerst M, Weber-Fahr W, Tunc-Skarka N, et al. Correlation of glutamate levels in the anterior cingulate cortex with self-reported impulsivity in patients with borderline personality disorder and healthy controls. Arch Gen Psychiatry. 2010;67:946–54.
33. Swick D, Jovanovic J. Anterior cingulate cortex and the Stroop task: neuropsychological evidence for topographic specificity. Neuropsychologia. 2002;40:1240–53.
34. Szbot CM, Eizirik M, Cunha RD, et al. Neuroimagem no transtorno de déficit de atenção/hiperatividade. Rev Bras Psiquiatr. 2001;23(Supl I):32–5.
35. Keller M, Werlang BSG. Flexibilidade na resolução de problemas em tentadores de suicídio. J Bras Psiquiatr. 2005;54:128–36.
36. Chang L, Ernst T, Witt MD, et al. Relationships among brain metabolites, cognitive function, and viral loads in antiretroviral-naïve HIV patients. Neuroimage. 2002;17:1638–48.
37. Li X, Lu ZL, D'Argembeau A, et al. The Iowa Gambling Task in fMRI images. Human Brain Mapp. 2010;31:410–23.
38. Vance DE. Implications of positive and negative neuroplasticity on cognition in HIV. Med Sci Monit. 2010;16:HY3–5.
1. PhD, Post-doctorate fellow, Program of Post-graduation in Medicine (Radiology), Universidade Federal do Rio de Janeiro (UFRJ), Rio de Janeiro, RJ, Brazil.
2. Psychologist, Fellow Master degree, Program of Post-graduation in Medicine (Radiology), Universidade Federal do Rio de Janeiro (UFRJ), Rio de Janeiro, RJ, Brazil.
3. MDs, Radiologists, Fellow Master degree, Program of Post-graduation in Medicine (Radiology), Universidade Federal do Rio de Janeiro (UFRJ), Rio de Janeiro, RJ, Brazil.
4. Student, Graduation Course of Medicine, Universidade Federal do Rio de Janeiro (UFRJ), Rio de Janeiro, RJ, Brazil.
5. Master, Fellow PhD degree, Program of Post-graduation in Medicine (Radiology), Universidade Federal do Rio de Janeiro (UFRJ), Medical Physicist at Clínica de Diagnóstico Por Imagem (CDPI), Rio de Janeiro, RJ, Brazil.
6. Medical Physicist at Clínica de Diagnóstico Por Imagem (CDPI), Rio de Janeiro, RJ, Brazil.
7. Post-doctorate, Associate Professor, Department of Radiology, Universidade Federal do Rio de Janeiro (UFRJ), Head of the Unit of Radiodiagnosis, Hospital Universitário Clementino Fraga Filho da Universidade Federal do Rio de Janeiro (HUCFF-UFRJ), MD, Radiologist at Clínica Menezes da Costa, Rio de Janeiro, RJ, Brazil.
8. Post-doctorate, Associate Professor, School of Psychology, Coordinator for the Group of Clinical and Experimental Neuropsychology, Pontifícia Universidade Católica do Rio de Janeiro (PUC-Rio), Rio de Janeiro, RJ, Brazil, Post-doctorate fellow at the Neuroimaging Center of Universit de Montral, Quebec, Canada.
9. PhD, Associate Professor Department of Radiology, Universidade Federal do Rio de Janeiro (UFRJ), Coordinator for the Sector of Magnetic Resonance Imaging at Hospital Universitário Clementino Fraga Filho da Universidade Federal do Rio de Janeiro (HUCFF-UFRJ), MD, Neurologist, Clínica de Diagnóstico Por Imagem (CDPI), Rio de Janeiro, RJ, Brazil.
Mailing Address:
Dr. Emerson L. Gasparetto
Estrada da Barra da Tijuca, 1006, ap. 1106, Bloco 5
Barra da Tijuca. Rio de Janeiro
RJ, 22641-003, Brazil
E-mail: egasparetto@gmail.com
Received October 28, 2010.
Accepted after revision November 19, 2010.
* Study developed at the Unit of Radiodiagnosis of Hospital Universitário Clementino Fraga Filho da Universidade Federal do Rio de Janeiro (HUCFF-UFRJ), Rio de Janeiro, RJ, Brazil.
 Vol. 44 nº 1 - Jan. /Feb. of 2011
Vol. 44 nº 1 - Jan. /Feb. of 2011