Radiologia Brasileira - Publicação Científica Oficial do Colégio Brasileiro de Radiologia
AMB - Associação Médica Brasileira CNA - Comissão Nacional de Acreditação
 Vol. 43 nº 6 - Nov. / Dec. of 2010
Vol. 43 nº 6 - Nov. / Dec. of 2010
|
ORIGINAL ARTICLE
|
|
Use of diffusion tensor magnetic resonance imaging in the assessment of patterns of white matter involvement in patients with brain tumors: is it useful in the differential diagnosis?* |
|
|
Autho(rs): Vanessa Granado Alves Itagiba1; Rafael Borges2; Luiz Celso Hygino da Cruz Jr3; Andre Dietz Furtado4; Romeu Côrtes Domingues5; Emerson Leandro Gasparetto6 |
|
|
Keywords: Diffusion tensor imaging; White matter; Brain tumors. |
|
|
Abstract: INTRODUCTION
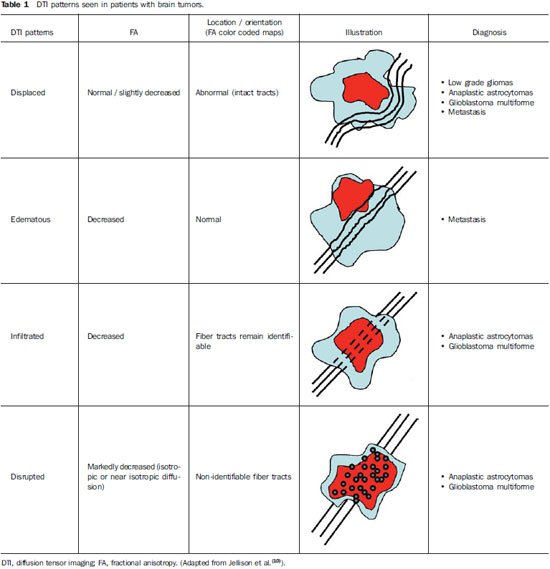
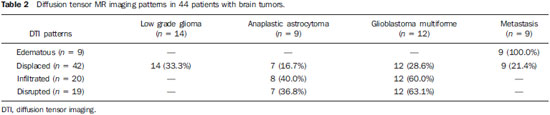
Magnetic resonance imaging (MRI) has been widely used in the evaluation of patients with brain tumors(1,2). However, in many cases, conventional MRI findings are not enough for accurately defining a differential diagnosis(1,3,4). Recently, advanced MRI techniques including spectroscopy, diffusion tensor imaging (DTI), perfusion and blood oxygen level dependent (BOLD) have added important information regarding not only the diagnosis of tumors, but also concerning treatment options, follow-up and prognosis(1,3,5). Nowadays, the DTI technique is achieving more importance in the study of patients with brain tumors(1,3,6). Diffusion tensor imaging can describe the direction of molecular water motion within tissues(1,3,7,8). In structures such as white matter fibers, the molecules diffusion will be more restricted perpendicular than parallel to the microstructural boundaries, which is called anisotropic diffusion(1,3,7,9). This information can generate color-coded schematic maps of white matter tracts, where the colors brightness is directly proportional to the fractional anisotropy (FA)(1,7). The DTI post-processing (tractography) allows the estimation of the anatomic relationship between the neoplasm and adjacent white matter tracts, allowing the maximization of tumor resection while minimizing the associated surgical morbidity(1,3). Some authors have suggested a classification of white matter involvement patterns in patients with brain tumors according to DTI findings, aiming at a better preoperative assessment of such lesions(1,2,6,10). They considered the following DTI patterns of white matter surrounding the tumors: edematous, displaced, infiltrated and disrupted(1,6,10). The classification of such different patterns was based on the FA values and on the direction and integrity of the white matter tracts adjacent to the tumors(1,2,6). To our knowledge, however, this classification has only be applied in small series of patients. The present study was aimed at evaluating the DTI findings in 44 patients with brain tumors submitted to preoperative MRI. We have attempted to reproduce, in a larger series of brain tumor patients, the different DTI patterns previously reported(1,6,10), aiming at evaluating the role of DTI for the assessment of white matter tracts involvement in patients with brain tumors as well as the utility of this technique for the differential diagnosis of brain tumors. MATERIALS AND METHODS Subjects The cohort study included 44 consecutive patients with intracranial neoplasms (24 male and 20 female patients; age range, 3–88 years; mean age, 44 years), who underwent MRI for pre-surgical evaluation. The final diagnoses of the tumors were based on the histological evaluation of material obtained either with surgical resection (n = 37) or stereotactic biopsy (n = 7). The series included low-grade gliomas (n = 14), glioblastoma multiforme (n = 12), anaplastic astrocytomas (n = 9), and metastases (n = 9). All patients signed a term of free and informed consent and the study was approved by the Committee for Ethics in Research of the institution. MRI acquisition The MRI studies were performed in a 1.5 T system (Avanto; Siemens Medical Systems, Erlangen, Germany) using a 8-channel head coil. All patients underwent a conventional MRI protocol, including the following sequences: coronal T2-weighed images (TR: 4410 ms, TE: 98 ms, FOV: 240 mm, matrix: 320 Χ 320 and 3-mm section thickness with 30% of interval), axial FLAIR images (TR: 9950 ms, TE: 100 ms, FOV: 220 mm, matrix: 256 Χ 256 and 5-mm section thickness with 35% of interval), as well as axial and sagittal T1-weighed images (TR: 37 5ms, TE: 11 ms, FOV: 240 mm, matrix: 212 Χ 256 and 5-mm section thickness with 30% of interval) before and after intravenous administration of 0.1 mmol/kg of gadolinium. DTI acquisition The DTI was performed using a single-shot echo-planar sequence with acceleration factor of two and with the following parameters: TR: 3100 ms, TE: 90 ms, FOV: 250 Χ 250 mm, matrix: 192 Χ 192, section thickness: 5 mm, interslice gap: 1.5 mm, bandwidth: 1346 kHz, EPI factor: 128, echo-spacing: 0.83, flip angle: 90º, NEX: 3, diffusion encoding in 12 different directions and b values = zero and 1000 s/mm2. DTI post-processing and analysis All images were transferred to a workstation (Leonardo; Siemens Medical Solutions, Erlangen, Germany) and post-processed with the software DTI Task Card (MGH-Martino’s Center; Boston, USA). Values for b, FA, and FA color-coded maps were automatically calculated according to protocols previously described in the literature(7). The FA color-coded map was generated by mapping the major eigenvector x, y, and z components into red, green, and blue color channels, which were weighted by FA. On the color maps, by standard definition, white matter tracts depicted in red have a right/left orientation, green tracts, anterior/posterior orientation, and blue tracts, superior-inferior orientation(1). We defined the FA as the anisotropy index to allow comparison with other studies, considering that most of them have used this index(11–14). For the images analysis, the white matter tracts adjacent to the tumors were visually compared with the corresponding contralateral tracts, and then characterized as follows (Table 1): a) displaced if they maintained normal or mildly decreased anisotropy as related to the corresponding tract on the contralateral hemisphere, but were situated at an abnormal location and/or orientation on the FA color-coded orientation maps; b) edematous if they showed reduced anisotropy, but maintained normal location and orientation on the FA color-coded maps; c) infiltrated if they demonstrated reduced anisotropy, remained identifiable on color-coded orientation maps, but exhibited altered color hues on the directional color maps, not attributable to bulk mass displacement; b) disrupted if color-coded anisotropy was markedly reduced, such that the tract could not be identified on orientation maps(1,6). Firstly, two experienced neuroradiologists analyzed all the DTI findings before evaluating the structural MR images and consensually reached final decisions regarding the different patterns of white matter involvement. RESULTS The most common DTI findings observed in this series of patients with brain tumors are shown on Table 1. In patients with low-grade gliomas, only the displaced pattern (14/14; 100%) was seen (Figure 1). The cases of anaplastic astrocytoma demonstrated the infiltrated (8/9; 88.9%), displaced (7/9; 77.8%) and disrupted (7/9; 77.8%) patterns, usually in association (Figure 2). All the patients with glioblastoma multiforme showed both the displaced, disrupted and infiltrated patterns (12/12; 100%) (Figure 3). As regards the patients with metastasis, all of them demonstrated the displaced and edematous patterns (9/9; 100%) (Figure 4). 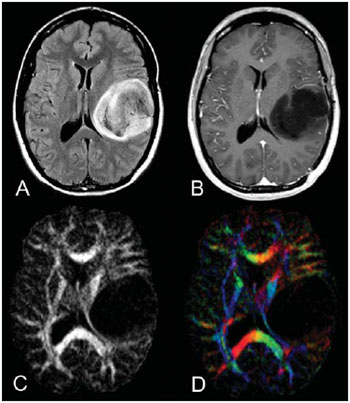 Figure 1. 32-year-old female patient with a low-grade glioma in the left frontotemporal region. On A, axial FLAIR shows a well-circumscribed mass with heterogeneous high-signal intensity, located in the left fronto-temporal region. On B, axial, post-contrast T1-weighted image demonstrates unenhanced lesion. On C, FA map shows the posterior limb of internal capsule medially displaced. On D, axial directional color map demonstrates normal anisotropy and abnormal location of the posterior limb of internal capsule (displaced pattern). 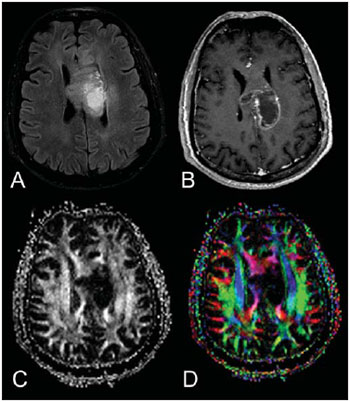 Figure 2. 49-year-old female patient with an anaplastic astrocytoma in the left centrum semiovale with extension towards the corpus callosum. On A, axial FLAIR shows a heterogeneous hyperintense mass involving the body of the corpus callosum. On B, axial post-contrast T1-weighted image demonstrates peripheral enhancement of the tumor. On C, FA map shows significant reduction of the FA in the body of the corpus callosum. Displacement of the left corona radiata is also seen. On D, axial directional color map demonstrates complete destruction of corpus callosum fibers (disrupted pattern). The corona radiata in the left hemisphere is displaced (displaced pattern) and shows both reduced anisotropy and disorientation of white matter fibers (reflected in asymmetric color hues) (infiltrated pattern). 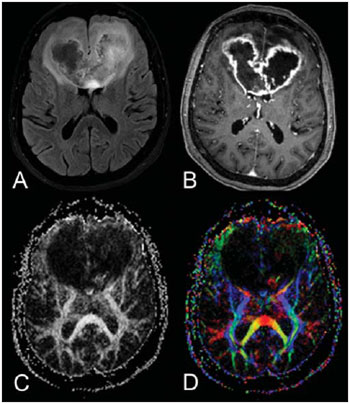 Figure 3. 62-year-olf male with a glioblastoma multiforme involving the corpus callosum and both frontal lobes (butterfly pattern). On A, axial FLAIR shows a heterogeneous mass involving the genu of the corpus callosum and both frontal lobes, surrounded by an area of high-signal intensity (peritumoral edema/ infiltration). On B, axial, post-contrast T1-weighted image demonstrates peripheral enhancement of the tumor. On C, FA map shows diffuse low-signal intensity in the genus of the corpus callosum and white matter in both frontal lobes. On D, axial directional color map shows complete destruction of the genu of the corpus callosum and the white matter tracts of the frontal lobes (disrupted pattern). The anterior limbs of the internal capsules are displaced (displaced pattern) and both present decreased anisotropy and disorientation of white matter fibers (reflected on asymmetric color hues) (infiltrated pattern). 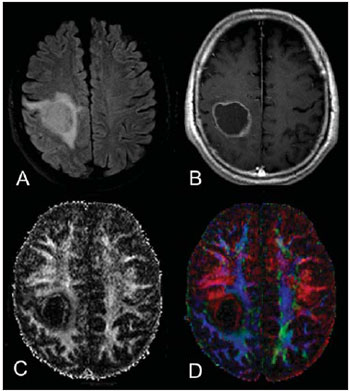 Figure 4. 59-year-old male patient with lung metastasis in the right frontopariental region. On A, axial FLAIR shows a hyperintense mass in the right frontoparietal region, surrounded by vasogenic edema. On B, axial, post-contrast T1-weighted image demonstrates peripheral enhancement of the lesion. On C, fractional anisotropy map demonstrates decreased anisotropy in the white matter surrounding the tumor. On D, axial directional color map shows decreased anisotropy in the area surrounding the metastasis (reflected by diminished color brightness) but with normal location and orientation (reflected by normal hues as compared with the homologous area in the contralateral hemisphere) (edematous pattern). In addition, there is a medial deviation of the corona radiata (displaced pattern). Alternatively, comparing the different DTI patterns with each histological diagnosis, there is a significant overlap between patterns, since different tumors demonstrated the same DTI patterns of adjacent white matter involvement (Table 2). The displaced pattern (n = 42) was observed in cases of low-grade gliomas (33.3%), glioblastoma multiforme (28.6%), metastasis (21.4%) and anaplastic astrocytoma (16.7%). The disrupted pattern (n = 19) was found in patients with glioblastoma multiforme (63.2%) and anaplastic astrocytoma (36.8%). Finally, the infiltrated pattern (n = 20) was demonstrated in cases of glioblastoma multiforme (60%) and anaplastic astrocytoma (40%). DISCUSSION The preoperative identification of the extent of malignant cell infiltration within white matter tracts constitutes a significant challenge. Tumor cells can invade and change the white matter fibers structure by widening, displacing, and/or disrupting the fiber bundles(15). The surgical treatment for brain tumors is aimed at achieving the maximum possible resection, while minimizing the neurological deficits resulting from surgical injury to the intact, functioning brain not affected by the tumor. This requires pre- and intraoperative mapping of the tumor as well as the definition of its relationship with functional structures, so that such structures can be preserved during surgical resection(10). In the present study, we evaluated the DTI findings in 44 patients with brain tumors who underwent preoperative MRI with the objective of describing the patterns of fiber tracts involvement by these tumors. Our results showed the previously described DTI patterns of white matter involvement, namely, edematous, displaced, infiltrated and disrupted(1,6). A significant overlap was observed between the different DTI patterns and the most common tumor types. The disrupted pattern was found in anaplastic astrocytomas and glioblastoma multiforme, while the displaced pattern was seen in metastases, low and high-grade gliomas; and the edematous pattern was observed only in cases of metastases. Conventional MRI cannot satisfactorily demonstrate relevant anatomical details and does not provide enough information about fiber bundles location and integrity(15,16). Recently, advanced MRI techniques, including spectroscopy, DTI, perfusion and BOLD, have added important information regarding not only the diagnosis of the tumors, but also concerning treatment options, follow-up and prognosis(1,3,5,9). The study of white matter tracts with magnetic resonance DTI allows the noninvasive investigation of neuronal fibers, based on the imaging characteristics of anisotropic water diffusion. The anisotropy of water diffusion in the brain white matter is a result of its organization into myelinated axon fibers, where diffusion is faster parallel than perpendicular to the fibers tract(1,7,15,17). Previous studies have assessed the DTI findings in patients with brain tumors, aiming at evaluating different patterns of white matter involvement(1,6,18). The suggested classification considered the FA values, integrity and orientation of white matter tracts on FA color-coded maps. The tracts were rated as displaced in cases of normal or slightly decreased anisotropy, situated at an abnormal location and/or orientation. The infiltrated pattern was associated with decreased anisotropy, without displacement of the normal white matter architecture, remaining identifiable on color-coded FA maps, but with abnormal color hues. The tracts with reduced anisotropy but normal location and/or orientation were called edematous. The infiltrated and edematous patterns seem to be associated with infiltration of white matter tracts by tumor and/or edema, and the differentiation between them is not always possible. Both patterns were characterized by substantially decreased FA but, in most of the cases, they differed in their appearance on directional color maps(6). Whereas the edematous pattern tracts were normal in location and orientation (showed normal color hues on the color maps), the infiltrated pattern tracts exhibited abnormal hues not attributable to bulk mass displacement, which reflects a more severe form of disorganization(6,10,19). Finally, the cases with markedly reduced anisotropy and unidentifiable tracts on directional color maps were rated as disrupted. However, the small series included in such studies limit the clinical application of these patterns of involvement of the white matter surrounding brain tumors. Witwer et al.(1) have studied DTI findings in nine patients with brain tumors, applying the previously mentioned DTI classification. They evaluated six cases of low-grade gliomas, two of high-grade gliomas and one case of metastasis. The authors found the displaced pattern only in patients with low-grade gliomas. The edematous pattern was showed in one case of high-grade glioma and in the patient with metastasis. The infiltrated and the disrupted patterns were found in one case of high- and in one of low-grade gliomas, respectively. Later, the same group of authors published 13 cases of brain tumors (primary and secondary; low- and high-grade) evaluated with a similar approach(6). The results were almost identical to the ones observed in the previous study, with the displaced and edematous patterns being seen in both malignant and benign tumors, the infiltrated pattern being demonstrated in infiltrating gliomas and the disrupted pattern being observed in both low- and high-grade tumors. In our series, we found conflicting results when comparing with the results reported by Witwer et al.(1). The displaced pattern was non-specific, since it was found in cases of metastasis, low- and high-grade gliomas. The edematous pattern was showed only in cases of metastasis, differing from previous results demonstrating this pattern also in cases of high-grade gliomas. In addition, the infiltrated and disrupted patterns, previously demonstrated in patients with both low- and high-grade gliomas, were seen only in cases of high-grade gliomas. In our series, the cases of low-grade gliomas presented only the displaced pattern; however, this pattern is not specific for these tumors, since it was already described in previous series evaluating other tumors such as anaplastic astrocytomas, glioblastoma multiforme and cerebral metastasis(1,6). All the nine cases of metastasis showed both the displaced and edematous patterns. As already mentioned, the displaced pattern was also observed in low and high-grade gliomas, which makes this pattern nonspecific. Although the results of the present study have demonstrated that the edematous pattern was only seen in metastasis, this pattern was also previously described in cases of gliomas(1,6). In our study, the disrupted pattern was shown only in high-grade gliomas; however, it has been previously described(1) in one case of low-grade glioma. In our series, some DTI patterns presented a good correlation with the histological diagnoses, such as the edematous pattern seen in the metastases and the infiltrated and disrupted patterns observed only in high-grade gliomas, which could suggest that the DTI might be able to distinguish high- from low-grade gliomas and metastases. Although our data have been relevant, we observed that there was a significant overlap between the different DTI patterns and the final diagnoses, which does not allow the differential diagnosis of most of these tumors using the DTI patterns of brain white matter involvement. Our study has several limitations. Although initial reports have suggested DTI advantages in the pre-surgical planning in cases of brain tumors, such reports as well as our results reflect preliminary conclusions. Another problem is the DTI sequence susceptibility to artifacts that could cause images distortion. Additionally, the definition of the different patterns of white matter involvement is basically visual and, thus, susceptible to interobserver disagreement. Finally, although we have presented one of the largest series evaluating brain tumors with DTI, the number of subjects enrolled in the study is still small, especially when one looks at the number of patients in the different subgroups. CONCLUSION The diffusion tensor imaging patterns of white matter tracts involvement assists in the mapping of white matter tracts adjacent to tumors and provide relevant information about tumors extent. Although we have observed that these patterns, such as the edematous pattern seen in metastases and the infiltrated and disrupted patterns in high-grade gliomas, may be useful in the differential diagnosis of some neoplasms, a significant overlap was observed between the patterns and the final histological diagnoses as our results are compared with previous studies. Therefore, in agreement with previous studies, we suggest that, although the DTI plays a relevant role in the preoperative evaluation of patients with brain tumors, this method is not useful in the differential diagnosis of these tumors. In spite of some DTI limitations, this technique is gaining support as a preoperative MRI method for evaluating brain tumors closely related to eloquent areas, optimizing the surgical strategies. Nevertheless, in the future, with new technical developments, DTI may become an important tool to predict the difference between these types of lesions. REFERENCES 1. Witwer BP, Moftakhar R, Hasan KM, et al. Diffusion-tensor imaging of white matter tracts in patients with cerebral neoplasm. J Neurosurg. 2002;97:568–75. 2. Yu CS, Li KC, Xuan Y, et al. Diffusion tensor tractography in patients with cerebral tumors: a helpful technique for neurosurgical planning and postoperative assessment. Eur J Radiol. 2005;56:197–204. 3. Sundgren PC, Dong Q, Gómez-Hassan D, et al. Diffusion tensor imaging of the brain: review of clinical applications. Neuroradiology. 2004;46:339–50. 4. Beppu T, Inoue T, Shibata Y, et al. Measurement of fractional anisotropy using diffusion tensor MRI in supratentorial astrocytic tumors. J Neurooncol. 2003;63:109–16. 5. Holodny AI, Ollenschleger MD, Liu WC, et al. Identification of the corticospinal tracts achieved using blood-oxygen-level-dependent and diffusion functional MR imaging in patients with brain tumors. AJNR Am J Neuroradiol. 2001;22:83–8. 6. Field AS, Alexander AL, Wu YC, et al. Diffusion tensor eigenvector directional color imaging patterns in the evaluation of cerebral white matter tracts altered by tumor. J Magn Reson Imaging. 2004;20:555–62. 7. Melhem ER, Mori S, Mukundan G, et al. Diffusion tensor MR imaging of the brain and white matter tractography. AJR Am J Roentgenol. 2002;178:3–16. 8. Laundre BJ, Jellison BJ, Badie B, et al. Diffusion tensor imaging of the corticospinal tract before and after mass resection as correlated with clinical motor findings: preliminary data. AJNR Am J Neuroradiol. 2005;26:791–6. 9. Inoue T, Ogasawara K, Beppu T, et al. Diffusion tensor imaging for preoperative evaluation of tumor grade in gliomas. Clin Neurol Neurosurg. 2005;107:174–80. 10. Jellison BJ, Field AS, Medow J, et al. Diffusion tensor imaging of cerebral white matter: a pictorial review of physics, fiber tract anatomy, and tumor imaging patterns. AJNR Am J Neuroradiol. 2004;25:356–69. 11. Lu S, Ahn D, Johnson G, et al. Peritumoral diffusion tensor imaging of high-grade gliomas and metastatic brain tumors. AJNR Am J Neuroradiol. 2003;24:937–41. 12. Tsuchiya K, Fujikawa A, Nakajima M, et al. Differentiation between solitary brain metastasis and high-grade glioma by diffusion tensor imaging. Br J Radiol. 2005;78:533–7. 13. Lu S, Ahn D, Johnson G, et al. Diffusion-tensor MR imaging of intracranial neoplasia and associated peritumoral edema: introduction of the tumor infiltration index. Radiology. 2004;232:221–8. 14. Sinha S, Bastin ME, Whittle IR, et al. Diffusion tensor MR imaging of high-grade cerebral gliomas. AJNR Am J Neuroradiol. 2002;23:520–7. 15. Stadlbauer A, Nimsky C, Buslei R, et al. Diffusion tensor imaging and optimized fiber tracking in glioma patients: histopathologic evaluation of tumor-invaded white matter structures. Neuroimage. 2007;34:949–56. 16. Price SJ, Jena R, Burnet NG, et al. Improved delineation of glioma margins and regions of infiltration with the use of diffusion tensor imaging: an image-guided biopsy study. AJNR Am J Neuroradiol. 2006;27:1969–74. 17. Puig J, Pedraza S, Blasco G, et al. Wallerian degeneration in the corticospinal tract evaluated by diffusion tensor imaging correlates with motor deficit 30 days after middle cerebral artery ischemic stroke. AJNR Am J Neuroradiol. 2010;31:1324–30. 18. Price SJ, Peña A, Burnet NG, et al. Tissue signature characterisation of diffusion tensor abnormalities in cerebral gliomas. Eur Radiol. 2004;14:1909–17. 19. Wang S, Kim S, Chawla S, et al. Differentiation between glioblastomas and solitary brain metastases using diffusion tensor imaging. Neuroimage. 2009;44:653–60. 1. Master Fellow degree, MD, Radiologist at CDPI Clínica de Diagnóstico Por Imagem, Hospital Central do Exército and Universidade Federal do Rio de Janeiro (UFRJ), Rio de Janeiro, RJ, Brazil. 2. MD, Radiology Resident, Universidade Federal do Rio de Janeiro (UFRJ), Rio de Janeiro, RJ, Brazil. 3. Fellow PhD degree, MD, Radiologist at CDPI Clínica de Diagnóstico Por Imagem, Clínica Multi-Imagem e Universidade Federal do Rio de Janeiro (UFRJ), Rio de Janeiro, RJ, Brazil. 4. MD, Radiologist, Clinical Fellow in Neuroradiology at Children’s Hospital of Pittsburgh, Pittsburgh, PA, USA. 5. MD, Radiologist, Director, CDPI - Clínica de Diagnóstico Por Imagem and Clínica Multi-Imagem, Rio de Janeiro, RJ, Brazil. 6. PhD, Associate Professor, Universidade Federal do Rio de Janeiro (UFRJ), MD, Radiologist at CDPI - Clínica de Diagnóstico Por Imagem and Clínica Multi-Imagem, Rio de Janeiro, RJ, Brazil. Mailing Address: Dr. Emerson L. Gasparetto Avenida das Américas, 4666, sala 325, Barra da Tijuca Rio de Janeiro, RJ, Brazil, 22640-102 E-mail: egasparetto@gmail.com * Study developed at CDPI Clínica de Diagnóstico Por Imagem and Clínica Multi-Imagem, Rio de Janeiro, RJ, Brazil. |
|
Av. Paulista, 37 - 7° andar - Conj. 71 - CEP 01311-902 - São Paulo - SP - Brazil - Phone: (11) 3372-4544 - Fax: (11) 3372-4554