Radiologia Brasileira - Publicação Científica Oficial do Colégio Brasileiro de Radiologia
AMB - Associação Médica Brasileira CNA - Comissão Nacional de Acreditação
 Vol. 43 nº 6 - Nov. / Dec. of 2010
Vol. 43 nº 6 - Nov. / Dec. of 2010
|
ORIGINAL ARTICLE
|
|
Evaluation of portal blood flow in schistosomal patients: a comparative study between magnetic resonance imaging and Doppler ultrasonography* |
|
|
Autho(rs): Alberto Ribeiro de Souza Leão1; Danilo Moulin Sales1; José Eduardo Mourão Santos2; Edson Nakano1; David Carlos Shigueoka3; Giuseppe D’Ippolito4 |
|
|
Keywords: Portal blood flow; Magnetic resonance imaging; Doppler ultrasonography; Reproducibility; Portal hypertension. |
|
|
Abstract: INTRODUCTION
In healthy patients, portal hepatic circulation can accommodate great variations in blood flow with small changes in portal pressure(1). The main hemodynamic alterations of this system are a chronic increase in venous pressure in the portal territory, defined as portal hypertension. This is usually secondary to splenic venous blood flow interference, and clinically translates into collateral circulation, visible as abdominal wall collaterals, ascites, and esophagogastric alterations, specifically esophageal varices, gastric varices, and congestive gastropathy(1,2). The gradient of portal pressure is the difference between the pressure in the inferior vena cava and the portal vein, and has a normal value of < 6 mmHg. When it increases above 10–12 mmHg, portal hypertension complications may occur(3). Disorders that may progress to portal system hypertension includes cirrhosis, and the hepatosplenic form of infection by Schistosoma mansoni, along with hepatic, biliary tract, or pancreatic neoplasms. Thromboembolic events in the portal vein, and suprahepatic disorders such as right heart failure or inferior vena cava occlusion by thrombi or tumors can also contribute(4). Upper digestive tract hemorrhage caused by esophagogastric varices is the main complication of portal hypertension in both cirrhotic and schistosomal patients, and has high morbimortality indices(5). The bleeding is a consequence of a chain of events, beginning with an increase in portal pressure, and progressing to the development and progressive dilation of gastroesophageal varices(6). Portal hypertension affects between 2% and 7% of patients with schistosomiasis, and is the main cause of digestive hemorrhages(7). The incidence of esophageal varices is approximately 85% in these patients, and progresses to bleeding in about two-thirds of all cases(8). The mortality rate at the first bleeding episode is 11.7%(8). The diagnosis of portal hypertension can be made by noninvasive methods, which include semiological data and complementary methods, and also by invasive methods including a direct approach of surgical measurement of the pressure in the portal system, or indirect methods that measure the wedged and free hepatic venous pressures, obtaining a hepatic venous pressure gradient between these two pressures(9). The direct measurement of portal pressure is the most accurate for evaluating its actual increase(9). The measurement of portal pressure levels may aid in the differential diagnosis of portal hypertension causes; in the evaluation of bleeding risk due to gastroesophageal varices rupture, which is the main cause of morbimortality; in the assessment of drug therapy efficacy; in prophylaxis of gastroesophageal varices bleeding; in therapeutic decisions in cases of hepatic resection; and in the evaluation of disease prognosis(5,9). In spite of the unquestionable advantages of pressure gradient measurement, this method is invasive and not widely available because of its high cost and operator-dependence. Therefore, the challenge of identifying a noninvasive marker for portal hypertension remains. Several authors have suggested that some parameters of Doppler ultrasonography (DUS) might be of prognostic value, and may be useful in assessing the risk of esophageal varices bleeding. However, the technique is not often used for this purpose, and its clinical usefulness is under debate(9). Noninvasive measurement of portal vein flow volume in patients with portal hypertension has gained acceptance as an alternative method for diagnosis and follow-up. Magnetic resonance imaging (MRI) is gaining acceptance as a noninvasive imaging method for evaluating hemodynamic parameters, including diagnostic approach and follow-up of patients with portal hypertension, including those cases of schistosomal origin. The high reproducibility of MRI for the evaluation of hepatic and splenic morphology in chronic schistosomal patients(10), and its role in the diagnostic differentiation of cirrhotic hepatopathy of alcoholic and viral origins, has been established(11,12). MRI has also been demonstrated as a reliable method for portal flow quantification in healthy patients, with better interobserver agreement than DUS, although the intermethod agreement for the quantification of portal flow is poor(13). The same reproducibility indices were observed in evaluating findings for periportal fibrosis, making MRI a comprehensive and accurate method in the evaluation of schistosomal patients(14). Diagnostic accuracy is a fundamental parameter for the usefulness of a diagnostic method, and this accuracy can be determined by measuring its reproducibility or interobserver agreement(15). This is necessary for validating noninvasive methods capable of evaluating hemodynamic parameters that may be pathologically modified in patients with portal hypertension. Therefore, patients with hepatosplenic schistosomiasis have been studied as a model for the evaluation of portal hypertension(11,12). The objective of this study was to evaluate the agreement between DUS and MRI, and the interobserver reproducibility of DUS and MRI for the quantification of portal flow volume in schistosomal patients. MATERIALS AND METHODS A prospective, cross-sectional, observational, double-blinded, and self-paired study was conducted from February 2005 to July 2007 on 21 patients (9 men and 12 women) ranging from 23–57 years (mean age, 40.9 years). This study was approved by the Institution’s Committee for Ethics in Research. Exams were performed at a maximum interval of 15 days, and were preferably conducted on the same day. Inclusion criteria were over 18 years old and Schistosoma mansoni infection diagnosis by rectal biopsy or strong clinical and laboratory evidence (signs of portal hypertension and/or positive stool ova and parasite exam) with positive epidemiological evidence (contact with pond or river water in endemic areas). Exclusion criteria were: contraindication for MRI (cardiac pacemaker, cochlear implant, claustrophobia, presence of cerebral aneurysm clips, allergy to paramagnetic contrast medium); history of alcoholism (ingestion of > 160 g of ethanol per week); positive serology for B or C hepatitis virus; history of proven autoimmune disorder that might progress as autoimmune hepatitis; splenectomized patients; irregular use of beta-blocker drugs (propranolol), so measurements would be made under drug influence; or total portal vein occlusion (total portal thrombosis). Cases of partial occlusion were not excluded, as cross-sectional areas of the vessel could be defined without compromising the portal flow volume measurement. MRI studies were performed with a Magnetom Sonata (Siemens; Erlangen, Germany) operating with a high magnetic field (1.5 T), a gradient of 40 mT/m, and a body coil for signal transmission/reception (phased array coil). DUS studies were performed with an EnVisor (Philips Medical Systems; Bothell, WA, USA), using a convex, multi-frequency transducer, following section planes standardized by the World Health Organization for sonographic evaluation of the liver, spleen, and splanchnic vascular system in schistosomal patients(16). Patients were evaluated after fasting for 6–8 hours for both imaging methods. For MRI studies, the patients were in dorsal decubitus position, with arms elevated above the head. To localize the portal vein, true fast imaging with steady precession sequences (TRUFI: TrueFISP) was performed in the coronal plane. The phase-contrast technique was used to measure portal flow. The technical parameters of the sequences are in Table 1. 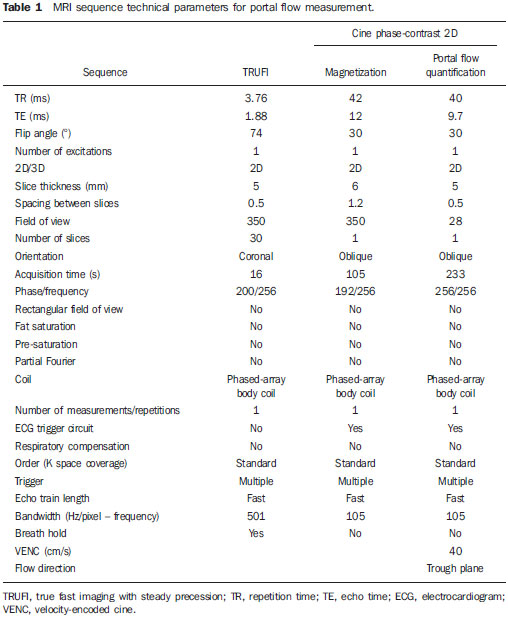 The imaging plane for flow mapping was perpendicularly adjusted to the middle segment of the portal vein (Figure 1). The time required for imaging ranged from 15 to 25 minutes. After exams, images were independently evaluated by two observers using a Leonardo (Siemens; Erlangen, Germany) workstation, with Argus software, for flow measurement and hemodynamic MRI studies (Figure 2). The acquired images package was manipulated so the perimeter for the section of the vessel was manually defined, and characterized by an area of higher signal intensity, with mean values of flow volume in the vessel, peak systolic velocity, mean velocity and section area supplied by the software. 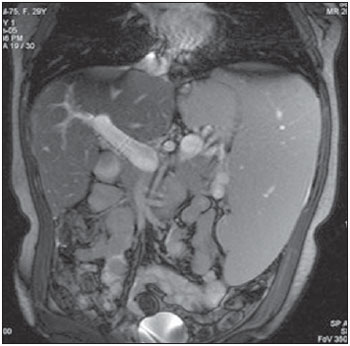 Figure 1. Coronal section plane of the abdomen acquired with the TRUFI sequence, used to define the middle segment of the portal vein. 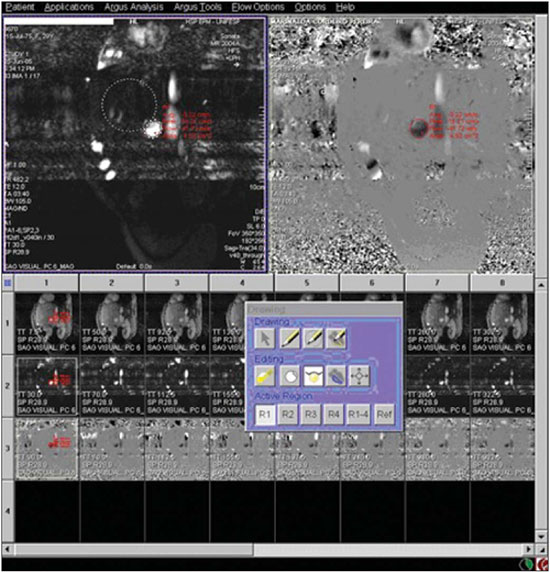 Figure 2 Images of the Leonardo workstation, which was used for the Argus software dedicated to the measurement of flow and MRI hemodynamic studies. Doppler study of the portal vein was performed with the patient in the dorsal decubitus position after a short rest, with oblique, subcostal and intercostal sections of the portal vein trunk at half-the-distance from its bifurcation, in a similar respiratory phase and with an insonation angle between 45° and 60° (Figure 3). Imaging took 20 to 30 minutes. The diameter of the portal vein was measured using calipers, in the same region where dopplefluxometric sampling was obtained for flow calculation. The selected interval of the spectral curve for analysis was at least four seconds. After entering required parameters, flow volumes were obtained. Calculations were based on Doppler spectral mapping and were automatically performed by the equipment (operator-independent)(17). 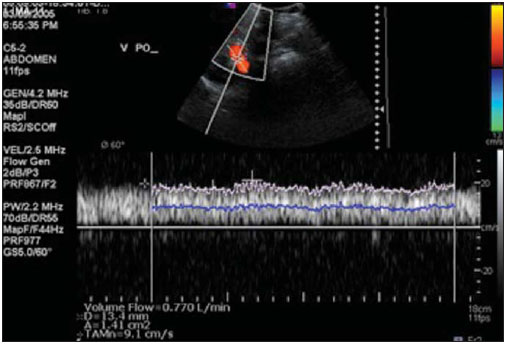 Figure 3 Spectral curve, with sample acquired by DUS, at the mid-point between the portal vein origin and bifurcation with an insonation angle of 60°. DUS imaging was performed and interpreted by three independent observers (observers 1, 2 and 3), with at least three years of experience in abdominal DUS after medical residency in imaging diagnosis. MRI studies were interpreted by two independent observers (observers 3 and 4, one with experience analyzing both methods), with at least five years of experience in abdominal MRI. For both methods, specific training for the measurement of portal flow was provided, with all observers agreeing upon the data collection methodology. Four statistical tools were used: Bland-Altman plots, paired t-tests, scatter-plots of the two measurements, and intraclass correlation coefficient (ICC) with a confidence interval (CI) of 95%. When combined, these tools offer complementary and useful information for analyzing intermethod reproducibility and interobserver agreement. The classification proposed by Fleiss (1981) was used for ICC interpretation (Table 2). 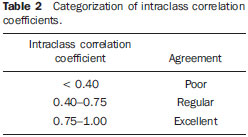 A statistical significance of 5% (α = 0.05) was adopted for all statistical analyses, with p-values < 5% (p < 0.05) considered significant. Statistical analysis was performed with SPSS 12.0 and MedCalc version 9.4.2.0 softwares. RESULTS Poor agreement between MRI and DUS (intermethod agreement) was observed. Nonetheless, interobserver reproducibility was excellent for magnetic resonance imaging evaluation and for Doppler ultrasonography, according to the results of all four observers. Correlation and Pearson coefficients for intermethod and interobserver correlation are in Table 3. 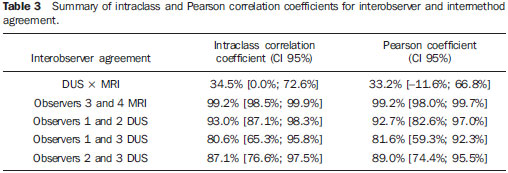 Based on the results of portal flow volume for the 21 schistosomal patients, the mean, median, and CI were calculated for each method. For DUS, the mean flow value ranged from 0.966 to 0.986 l/min with a median from 0.910 to 1.010 l/min, and a standard deviation between 0.464 and 0.590 l/min. For phase-contrast MRI, mean flow values ranged from 0.933 to 0.937 l/min with a median from 0.842 to 0.862 L/min, and a standard deviation between 0.570 and 0.575 l/min. DISCUSSION Portal hypertension leads to complications that tend to progress with the course of the disease. An increase in pressoric levels promotes an increase in varices size, and in tension on the vessels walls, causing bleeding that is associated with high morbimortality. Thus, the management of pressoric levels in the portal vein is essential(18). Recently, the management of hemodynamic parameters that reflect physiopathological changes that lead to bleeding has attracted interest. For example, no bleeding was found with portal vein pressoric levels < 12 mmHg(19). The most accurate technique for evaluating the severity of portal hypertension is catheterization of one of the hepatic veins, and measurement of the pressure at this point and at a free position. The hepatic venous pressure gradient is calculated as the difference between the pressure values. However, this is an expensive procedure with risks, particularly for patients a with limited life expectancy, such as patients with portal hypertension(20). Consolidating alternatives to invasive techniques for measuring portal pressure for diagnostic purposes, including endoscopic screening of gastroesophageal varices to prevent the risk of upper digestive bleeding, is a constant objective for improving the treatment of patients with portal hypertension(21). Currently, DUS is considered the method of choice for evaluating portal hypertension, because of its wide availability and low cost. However, DUS is susceptible to errors in measuring the cross-sectional area of vessel, depending of intra- and interobserver variability, certain physiological events and biotypes of patients(22–27). In healthy individuals, flow velocity is usually > 15 cm/s. In some studies, variation can be greater, with values ranging from 12 to 20 cm/s, and measurements higher than the upper threshold may be identified in healthy individuals. In cirrhotic patients, values usually range between 8–13 cm/s(22). In patients with hepatosplenic schistosomiasis, portal blood flow velocity values are usually within normal limits(22). Recently, MRI techniques have been used as noninvasive evaluation of the splanchnic venous system, and measurement of respective hemodynamic parameters, especially in follow-up of patients with portal hypertension(28). Studies using previously calibrated phantoms that simulate the physiological conditions of the portal venous system have demonstrated a high correlation between flow volume values with phase-contrast MRI(25,26). The main motivating factors for this study were the high correlation obtained in vitro by phase-contrast MRI evaluation of hemodynamic parameters in phantoms simulating the portal system. Also, no systematic study could be found in the medical literature on using MRI to measure hepatic and splenic hemodynamic variables in patients with schistosomiasis. MRI is useful not only for evaluating hemodynamic parameters, but also for comprehensive analysis of the abdominal venous system in patients with portal hypertension. Thus, MR angiography has already been demonstrated as useful for the evaluation of the collateral circulation that may be found in many of portal hypertension patients(23,29). The portal hypertension schistosomal model was selected because of the wide range and variability of observed flow volumes, typical of its hemodynamic pattern of portal hyperflow, which made it suitable for investigating and evaluating the accuracy of diagnostic methods(5). The imaging techniques used in this study are widely available for both MRI and DUS devices, so this work may be the basis for other investigations on other equipments. A poor intermethod agreement was observed. The ICC was 34.5% (CI 95% = 0.0%, 72.6%). Bland-Altman plots demonstrated a higher number of positive differences, i.e., DUS measurements with values higher than those from MRI. A plausible explanation for the poor intermethod agreement in portal vein flow measurements is the variation in volume as a function of the respiratory cycle phase. This parameter is difficult to obtain. In clinical practice, phase-contrast image acquisition with the breath-holding technique is not feasible because of the acquisition time. In these cases image acquisition uses the free-breathing technique, which is not used in Doppler ultrasonography. Wolf et al., in a study evaluating the influence of the respiratory cycle on variability in phase-contrast methods that are influenced by breathing, concluded the possibility of error, and suggested that the effect in clinical applications could be significant, and include flow measurement in vessels such as the inferior vena cava, pulmonary vessels and the portal vein(30). MRI reproducibility had a high interobserver agreement. The correlation between readings was classified as nearly perfect, with an ICC of 99.2% (CI 95% = 98.5%, 99.9%). These results are consistent with reports demonstrating a high MRI reproducibility in the evaluation of subjective and objective parameters(10–14,25–29,31–33). DUS reproducibility was evaluated at three different points, and readings from the three observers were combined in pairs. In these cases, the results from the three different analyses were very satisfactory, with a high ICC observed for all situations (r = 0.80, 0.87, 0.93). By paired t-test, observers were in agreement with the mean value, and statistical tests did not demonstrate significant differences in the acquired mean flow. A high correlation was seen between the measurements generated by the observers. Analysis of interobserver variability for quantitative variables demonstrated that the method can be used for objective evaluation of flow variations in patients with portal hypertension. This study evaluated MRI and DUS reproducibility for measurement of mean portal flow volume, and revealed a good interobserver agreement. This has never before been reported for schistosomal patients. Schistosomiasis is a prevalent disease in underdeveloped countries. The wide availability of DUS and the excellent cost-benefit ratio, as well as evidence of the good reproducibility of this diagnostic method reinforce its possibility in propedeutic and semiological approaches for patients with portal hypertension. Limitations in this study include the size of the sample (21 patients), and the knowledge by the observers that all cases were schistosomal patients. We could not perform direct measurements of hepatic venous pressure gradients, as this procedure is invasive and not used for routine clinical treatment in this group of patients. Thus, a reference standard for gauging the effectiveness of the portal flow measurements by DUS and MRI was not available. Therefore, knowing which measurement method was the most accurate was not possible, and high intermethod agreement was not observed. Studies on animal models are be required to demonstrate the accuracy of such measurements. In summary, this study demonstrated the high reproducibility of DUS and phase-contrast MRI for measurement of portal vein flow volume in patients with portal hypertension of schistosomal origin. We suggest using both methods for the evaluation of such variable. However, the absolute values for portal vein flow volume obtained by DUS were not comparable to those acquired by phase-contrast sequence, and we saw no intermethod agreement, possibly because of the variability associated with acquisition using either breath-holding or free-breathing. Further studies establishing the physiological and pathological values for portal flow volume for each method may define the usefulness of DUS and phase-contrast MRI in diagnostic and prognostic approaches of hemodynamic portal alterations. REFERENCES 1. Bem RS, Lora FL, Souza RCA, et al. Correlação das características do ecodoppler do sistema porta com presença de alterações endoscópicas secundárias à hipertensão porta em pacientes com cirrose hepática. Arq Gastroenterol. 2006;43:178–83. 2. Assef JC, Vieira ACPO, Saito HCG, et al. Modelo experimental de formação de varizes esofágicas por hipertensão portal esquistossomótica em hamsters. Rev Col Bras Cir. 2005;32:209–13. 3. Rodríguez-Vilarrupla A, Fernández M, Bosch J, et al. Current concepts on the pathophysiology of portal hypertension. Ann Hepatol. 2007;6:28–36. 4. Petroianu A. Tratamento cirúrgico da hipertensão porta na esquistossomose mansoni. Rev Soc Bras Med Trop. 2003;36:253–65. 5. Alves A Jr, Fontes DA, de Melo VA, et al. [Schistosomal portal hypertension: influence of the portal blood flow in serum levels of hepatic enzymes]. Arq Gastroenterol. 2003;40:203–8. 6. Dell’era A, Bosch J. Review article: the relevance of portal pressure and other risk factors in acute gastro-oesophageal variceal bleeding. Aliment Pharmacol Ther. 2004;20 Suppl 3:8–17. 7. Melo-Júnior MR, Figueiredo JL, Araújo Filho JLS, et al. Hipertensão porta na esquistossomose mansônica: repercussões do tratamento cirúrgico no perfil histomorfométrico da mucosa gástrica. Rev Soc Bras Med Trop. 2007;40:71–5. 8. Ferraz AAB, Albuquerque PC, Lopes EPA, et al. The influence of periportal (pipestem) fibrosis on long term results of surgical treatment for schistosomotic portal hypertension. Arq Gastroenterol. 2003;40:4–10. 9. Dittrich S, Mattos AA, Cheinquer H, et al. Correlação entre a contagem de plaquetas no sangue e o gradiente de pressão venosa hepática em pacientes cirróticos. Arq Gastroenterol. 2005;42:35–40. 10. Bezerra ASA, D’Ippolito G, Caldana RP, et al. Avaliação hepática e esplênica por ressonância magnética em pacientes portadores de esquistossomose mansônica crônica. Radiol Bras. 2004;37:313–21. 11. Bezerra ASA, D’Ippolito G, Caldana RP, et al. Differentiating cirrhosis and chronic hepatosplenic schistosomiasis using MRI. AJR Am J Roentgenol. 2008;190:W201–7. 12. Bezerra ASA, D’Ippolito G, Caldana RP, et al. Chronic hepatosplenic schistosomiasis mansoni: magnetic resonance imaging and magnetic resonance angiography findings. Acta Radiol. 2007;48:125–34. 13. Costa JD, Leão ARS, Santos JEM, et al. Quantificação do fluxo portal em indivíduos sadios: comparação entre ressonância magnética e ultra-som Doppler. Radiol Bras. 2008;41:219–24. 14. Scortegagna Junior E, Leão ARS, Santos JEM, et al. Avaliação da concordância entre ressonância magnética e ultra-sonografia na classificação da fibrose periportal em esquitossomóticos, segundo a classificação de Niamey. Radiol Bras. 2007;40:303–8. 15. Winkfield B, Aubé C, Burtin P, et al. Inter-observer and intra-observer variability in hepatology. Eur J Gastroenterol Hepatol. 2003;15:959–66. 16. Niamey Working Group, 2000. Ultrasound in schistosomiasis: a practical guide to the standardized use of ultrasonography for the assessment of schistosomiasis-related morbidity. Second International Workshop, October 22–26, 1996, Niamey, Niger. 17. Widman A, Oliveira IRS, Speranzini MB, et al. Hipertensão portal por esquistossomose mansônica hepatoesplênica: efeito da desconexão ázigo-portal com esplenectomia no diâmetro e na velocidade média de fluxo do sistema portal (estudo ultra-sonográfico com Doppler). Arq Gastroenterol. 2001;38:19–23. 18. Vitális Z, Papp M, Tornai I, et al. [Prevention and treatment of esophageal variceal bleeding]. Orv Hetil. 2006;147:2455–63. 19. Dagher L, Burroughs A. Variceal bleeding and portal hypertensive gastropathy. Eur J Gastroenterol Hepatol. 2001;13:81–8. 20. Horn JR, Zierler B, Bauer LA, et al. Estimation of hepatic blood flow in branches of hepatic vessels utilizing a noninvasive, duplex Doppler method. J Clin Pharmacol. 1990;30:922–9. 21. Dib N, Konate A, Oberti F, et al. [Non-invasive diagnosis of portal hypertension in cirrhosis. Application to the primary prevention of varices]. Gastroenterol Clin Biol. 2005;29:975–87. 22. Machado MM, Rosa ACF, Barros N, et al. Estudo Doppler na hipertensão portal. Radiol Bras. 2004;37:35–9. 23. Paulson EK, Kliewer MA, Frederick MG, et al. Doppler US measurement of portal venous flow: variability in healthy fasting volunteers. Radiology. 1997;202:721–4. 24. Iwao T, Toyonaga A, Shigemori H, et al. Echo-Doppler measurements of portal vein and superior mesenteric artery blood flow in humans: inter- and intra-observer short-term reproducibility. J Gastroenterol Hepatol. 1996;11:40–6. 25. Burkart DJ, Johnson CD, Morton MJ, et al. Volumetric flow rates in the portal venous system: measurement with cine phase-contrast MR imaging. AJR Am J Roentgenol. 1993;160:1113–8. 26. Tsunoda M, Kimoto S, Hamazaki K, et al. Quantitative measurement of portal blood flow by magnetic resonance phase contrast: comparative study of flow phantom and Doppler ultrasound in vivo. Acta Med Okayama. 1994;48:283–8. 27. de Vries PJ, van Hattum J, Hoekstra JB, et al. Duplex Doppler measurements of portal venous flow in normal subjects. Inter- and intra-observer variability. J Hepatol. 1991;13:358–63. 28. Liu H, Cao H, Wu ZY. Magnetic resonance angiography in the management of patients with portal hypertension. Hepatobiliary Pancreat Dis Int. 2005;4:239–43. 29. Caldana RP, Bezerra ASA, D’Ippolito G, et al. Estudo da circulação hepatomesentérica pela angiografia por ressonância magnética com gadolínio: comparação entre doses simples e dupla no estudo de pacientes esquistossomóticos. Radiol Bras. 2006;39:243–51. 30. Wolf RL, Hangiandreou NJ, Felmlee JP, et al. Error in MR volumetric flow measurements due to ordered phase encoding in the presence of flow varying with respiration. Magn Reson Med. 1995;34:470–5. 31. Leão ARS, Santos JEM, Moulin DS, et al. Mensuração do volume de fluxo portal em pacientes esquistossomóticos: avaliação da reprodutibilidade do ultra-som Doppler. Radiol Bras. 2008;41:305–8. 32. Sales DM, Santos JEM, Shigueoka DC, et al. Correlação interobservador das alterações morfológicas das vias biliares em pacientes com esquistossomose mansoni pela colangiorressonância magnética. Radiol Bras. 2009;42:277–82. 33. Gonzalez TD, Santos JEM, Sales DM, et al. Avaliação ultra-sonográfica de nódulos sideróticos esplênicos em pacientes esquistossomóticos com hipertensão portal. Radiol Bras. 2008;41:69–73. 1. Masters, MDs., Radiologists, Universidade Federal de São Paulo (Unifesp), São Paulo, SP, Brazil. 2. PhD, MD, Radiologist, Universidade Federal de São Paulo (Unifesp), São Paulo, SP, Brazil. 3. PhD, Affiliate Professor, Universidade Federal de São Paulo (Unifesp), São Paulo, SP, Brazil. 4. Associate Professor, Universidade Federal de São Paulo (Unifesp), São Paulo, SP, Brazil. Mailing Address: Dr. Alberto Ribeiro de Souza Leão Rua Canário, 644, ap. 42, Moema São Paulo, SP, 04521-002, Brazil E-mail: ar.leao@uol.com.br Received November 15, 2009. Accepted after revision March 5, 2010. * Study developed at Department of Imaging Diagnosis – Universidade Federal de São Paulo (Unifesp), São Paulo, SP, Brazil. |
|
Av. Paulista, 37 - 7° andar - Conj. 71 - CEP 01311-902 - São Paulo - SP - Brazil - Phone: (11) 3372-4544 - Fax: (11) 3372-4554