Radiologia Brasileira - Publicação Científica Oficial do Colégio Brasileiro de Radiologia
AMB - Associação Médica Brasileira CNA - Comissão Nacional de Acreditação
 Vol. 43 nº 5 - Sep. / Oct. of 2010
Vol. 43 nº 5 - Sep. / Oct. of 2010
|
WHICH IS YOUR DIAGNOSIS?
|
|
Which is your diagnosis? |
|
|
Autho(rs): Marcelo Souto Nacif1; Radwa A.A. Noureldin2; Christopher T. Sibley3; Evrim B. Turkbey2; João A.C. Lima4; David A. Bluemke5 |
|
|
A 41-year-old female with heart failure was referred for evaluation by cardiac magnetic resonance imaging (MRI).
Images description Figure 1. ECG-gated cine-MRI acquisition in four chamber (A,B) and delayed enhancement in four chamber (C) and short axis (D). Systole (A) and diastole (B) showing a mild dilated cardiomyopathy and a mild tricuspid regurgitation. On the delayed enhancement images (C,D) there was no fibrosis or focal scar. 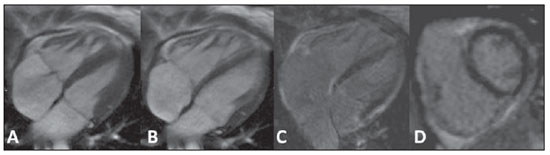 Figure 1. ECG-gated cine-MRI acquisition in four chamber (A,B) and delayed enhancement in four chamber (C) and short axis (D). Figure 2. ECG-gated MRI tagging (A,B,D,E) and look-locker T1 map (C,F). The tagging analysis (A,B,D,E) showed a reduced ejection fraction (EF = 27%). The peak circumferential strain in the midwall at the base, midcavity and apex were –16%, –13% and –11%. The T1 map analysis (C,F) showed a T1 time of 320 ms. Background: Normal ranges for midwall peak Ecc strain (%) is –0.23 ± 0.04(1). Normal volunteers data from our lab showed that 492.2 ± 44.7 ms (mean ± standard deviation) is the normal range for look-locker sequence at 15 minutes after gadolinium injection(2,3). 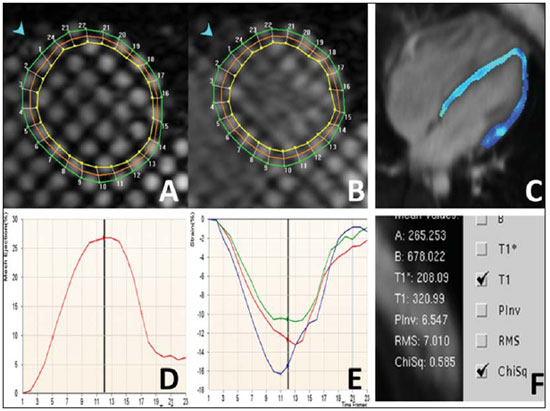 Figure 2. ECG-gated MRI tagging (A,B,D,E) and look-locker T1 map (C,F). Diagnosis: Heart failure with strain analysis and tissue characterization for fibrosis (T1 map). COMMENTS Heart failure (HF) is associated with significant morbidity, mortality, and financial burden to health care services. In the U.S., around 5,700,000 people, representing 2.5% of Americans older than 20 years of age, have been diagnosed with this condition. The aging of the population and the improved prognosis of patients with acute coronary events further fuel the HF epidemic. Consequently, hospital discharges increased for HF in United States and in Brazil(4,5). The early diagnosis and identification of the underlying etiology of HF is of paramount importance. Although the general treatment is common to many patients, some patient conditions require specific treatment and may be correctable(4). Cardiovascular magnetic resonance (CMR) is a rapidly evolving technology that is increasingly being used for the noninvasive imaging of the expanding HF population(4). Future developments in the care of advanced heart disease, including stem cell therapy, device therapy to control remodeling, and percutaneous valve interventions, as well as the need to identify subclinical heart disease, are likely to expand this use(6). CMR imaging Assessment of ventricular global and regional function by steady state free precession cine, fibrosis or scar by delayed enhancement imaging, flow volume and velocities by phase contrast, and also morphology by double and triple IR with or without fat suppression are established techniques(7). CMR tagging is a powerful non-invasive diagnostic tool for quantifying regional systolic and diastolic myocardial function(8–10). CMR tagging is able to reveal previously undetected components of regional myocardial mechanical function and, thus, aid in the early detection and management of a wide range of myocardial disease processes. Moreover, ongoing developments in the technology, imaging techniques, and analytical tools used to implement CMR tagging will improve the analyses of regional myocardial function(10). CMR methods may be useful in the quantification of fibrosis even in visual negative delayed enhancement imaging. The T1 time is shortened by fibrosis, and T1 mapping is a potentially quantifiable marker of the extent and severity of fibrosis when validated against biopsy samples and applied in clinical settings associated with nonischemic fibrosis(6). In practice, the present case reflects the T1 map relevance (Figure 3). The relaxation time T1 is short for an acquisition performed 10 minutes after gadolinium injection, demonstrating an increase in the extracellular space and the gadolinium permanence, with a slower washout. 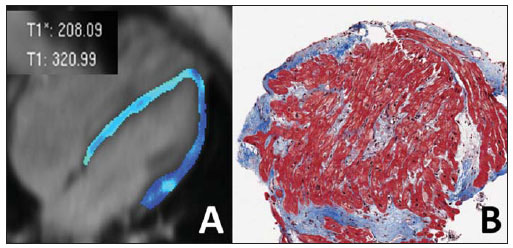 Figure 3. T1 map (A) showing short T1 time (320 ms) for the global left ventricle in the quantitative analysis, and T1 map showing blue color in the qualitative analysis (green is normal). Biopsy specimen of the same patient (B) showing in blue the fibrosis replacement and cardiac myocities disarrangement. Important considerations The successful application of these techniques will be determined not only by methodological progress but also by the integration of this information into clinical care. The latter requires not only rigorous clinical validation in prospective trials that adhere to evidence-based medicine criteria but also appropriate training of cardiovascular specialists in the cost efficient use of imaging technologies(6,7). Besides these clinical benefits, new imaging technologies will change cardiovascular research by providing unique tools to quantitatively study the disease process in animal models and humans. This will not only improve our understanding of the disease process but will also accelerate the evaluation of new drugs and their availability to patients(11). REFERENCES 1. Moore CC, Lugo-Olivieri CH, McVeigh ER, et al. Three-dimensional systolic strain patterns in the normal human left ventricle: characterization with tagged MR imaging. Radiology. 2000;214: 453–66. 2. Nacif MS, Turkbey EB, Gai N, et al. Myocardial T1 mapping with MRI: what is necessary to know about relationship of clinically used look-locker to MOLLI sequences. Am Heart J. [in press]. 3. Gai N, Turkbey E, Nazarian S, et al. T1 mapping of the gadolinium enhanced myocardium: adjustment for factors affecting inter-patient comparison. Magn Reson Med. [in press]. 4. Karamitsos TD, Francis JM, Myerson S, et al. The role of cardiovascular magnetic resonance imaging in heart failure. J Am Coll Cardiol. 2009;54: 1407–24. 5. Moutinho MA, Colucci FA, Alcoforado V, et al. Heart failure with preserved ejection fraction and systolic dysfunction in the community. Arq Bras Cardiol. 2008;90:132–7. 6. Marwick TH, Schwaiger M. The future of cardiovascular imaging in the diagnosis and management of heart failure, part 1: tasks and tools. Circ Cardiovasc Imaging. 2008;1:58–69. 7. American College of Cardiology Foundation Task Force on Expert Consensus Documents, Hundley WG, Bluemke DA, et al. ACCF/ACR/AHA/NASCI/SCMR 2010 expert consensus document on cardiovascular magnetic resonance: a report of the American College of Cardiology Foundation Task Force on Expert Consensus Documents. Circulation. 2010;121:2462–508. 8. Han Y, Chan J, Haber I, et al. Circumferential myocardial strain in cardiomyopathy with and without left bundle branch block. J Cardiovasc Magn Reson. 2010;12:2. 9. Leong DP, De Pasquale CG, Selvanayagam JB. Heart failure with normal ejection fraction: the complementary roles of echocardiography and CMR imaging. J Am Coll Cardiol Img. 2010;3: 409–20. 10. Shehata ML, Cheng S, Osman NF, et al. Myocardial tissue tagging with cardiovascular magnetic resonance. J Cardiovasc Magn Reson. 2009;11:55. 11. Marwick TH, Schwaiger M. The future of cardiovascular imaging in the diagnosis and management of heart failure, part 2: clinical applications. Circ Cardiovasc Imaging. 2008;1:162–70. 1. MD, PhD, Professor at Radiology Department, Faculdade de Medicina da Universidade Federal Fluminense (UFF), Niterói, RJ, Brazil, Fellow of Radiology and Imaging Sciences, National Institutes of Health (NIH) Clinical Center, Bethesda, MD, USA. Fellow of Cardiology, Johns Hopkins School of Medicine, Baltimore, MD, USA. 2. MD, Fellow of Radiology and Imaging Sciences, National Institutes of Health (NIH) Clinical Center, Bethesda, MD, USA. 3. MD, Staff Clinician, Department of Radiology and Imaging Sciences, National Institutes of Health (NIH) Clinical Center, Bethesda, MD, USA. 4. MD, Division of Cardiology, Johns Hopkins University School of Medicine, Baltimore, MD, USA. 5. MD, PhD, Director, Department of Radiology and Imaging Sciences, National Institutes of Health (NIH) Clinical Center, National Institute of Biomedical Imaging and Bioengineering, Bethesda, MD, USA. Mailing address: Dr. Marcelo Souto Nacif Cordell Avenue 4583 20814 Bethesda, MD, USA E-mail: msnacif@yahoo.com.br Web site: www.msnacif.med.br Study developed at Department of Radiology and Imaging Sciences, National Institutes of Health (NIH) Clinical Center, Bethesda, MD, USA |
|
GN1© Copyright 2024 - All rights reserved to Colégio Brasileiro de Radiologia e Diagnóstico por Imagem
Av. Paulista, 37 - 7° andar - Conj. 71 - CEP 01311-902 - São Paulo - SP - Brazil - Phone: (11) 3372-4544 - Fax: (11) 3372-4554
Av. Paulista, 37 - 7° andar - Conj. 71 - CEP 01311-902 - São Paulo - SP - Brazil - Phone: (11) 3372-4544 - Fax: (11) 3372-4554