Radiologia Brasileira - Publicação Científica Oficial do Colégio Brasileiro de Radiologia
AMB - Associação Médica Brasileira CNA - Comissão Nacional de Acreditação
 Vol. 43 nº 4 - July / Aug. of 2010
Vol. 43 nº 4 - July / Aug. of 2010
|
ORIGINAL ARTICLE
|
|
Spatial distribution analysis of absorbed dose in ocular proton radiation therapy |
|
|
Autho(rs): Marília Tavares Christóvão1; Tarcísio Passos Ribeiro de Campos2 |
|
|
Keywords: Proton therapy; Geant4; SISCODES; Ocular radiotherapy; Protons. |
|
|
Abstract: INTRODUCTION
Proton radiation therapy has been increasingly utilized by a growing number of international institutions. Several clinical and dosimetry studies have been developed with the objective of evaluating the benefits of such technique, particularly in the treatment of cancer patients. According to the statistics of the Particle Therapy Co-Operation Group (PTCOG), there are 30 facilities in operation, with 67,097 patients treated by proton radiation therapy, of which 18,055 patients with ocular tumors. By 2013, 22 new facilities are expected to be in operation, with approximately 70 new treatment rooms(1). The management of ocular neoplasms, particularly uveal melanomas, is amongst the main applications of proton radiation therapy. Such protocols preserve sensitive eye structures (optic nerve, cornea, lens, retina and crystalline lens), besides reducing the enucleation rate. Eyes preservation is achieved in 90% to 95% of cases, with vision remaining functional after 5 years in approximately 50% of the patients(2). Melanoma is a malignant tumor that originates in the pigmentation cells. In the eyes, the site with greater melanomas incidence of is the uvea (choroid), these tumors being the most common among the primary ocular tumors. In Europe, the yearly incidence of the disease achieves from 2 to 8 cases/million inhabitants(3), while in the United States the incidence achieves approximately 4.3 cases/million inhabitants(4). The evaluation of absorbed doses in the planning of ocular teletherapy is restricted to international centers that provide such treatment. The evaluation of absorbed dose in ocular structures exposed to proton beams has not been a frequent theme in the scientific literature. However, during any clinical or even comparative evaluation between different therapeutic approaches such as ophthalmic plaque brachytherapy and proton therapy, it is necessary to know the distribution of absorbed dose in ocular structures. Aiming at this purpose, the present study approaches dosimetry in simulated proton beam treatment on a human eye model. Results from computer simulations of proton therapy performed at a pre-existing facility are presented. Proton radiation therapy in the ocular region is recommended for the irradiation of tumors located in the posterior region to the ocular equator, with restriction to the regions where sensitive structure are located, such as the optical nerve, the lacrimal gland and the crystalline lens. The Geant4 (GEometry ANd Tracking) platform is of interest, as it evaluates the passage of charged heavy particles, such as protons or heavy ions, through matter. The Geant4 Toolkit, an open source software package, is composed of tools that can be utilized to simulate the passage of charged heavy particles through matter by the Monte Carlo method. Such method is widely recognized for its accuracy in the simulation of nuclear particle transport in complex geometries, such as voxel-based computational models and irradiation installations. The present study proposes the evaluation of depth-dose profiles and dose spatial distribution for ocular proton radiation therapy protocols in a typical installation, based on computational simulations utilizing the Geant4 and SISCODES (Computer System for Dosimetry in Radiotherapy) codes in a human eye model discretized into voxels. MATERIALS AND METHODS Protocol and irradiation installations taken as a model The present simulations reproduce a proton radiation therapy protocol, with data from an actual experiment of an operational installation comprising its main elements that applies proton beams for the management of ocular pathologies. Such installation is located at the Centro di AdroTerapia e Applicazioni Nucleari Avanzate (CATANA) of the Istituto Nazionale di Fisica Nucleare - Laboratori Nazionali del Sud (INFN-LNS), Catania, Italy - CATANA/INFN-LNS(5). Analysis software and graphic tools The software tool utilized in the present study is the Geant4 code. Interactive graphic tools (OpenGL) and data analysis tools (AIDA and JAS3) were also utilized in the simulation environment. The data resulting from the analysis tools (AIDA and JAS3) are generated during the simulation presenting the Bragg peak and relevant dose distributions originating from the protocol. Many of the parameters necessary for the protocol definition were obtained from preexisting processes available at the Geant4 library, denominated hadrontherapy. The Geant4 libraries on hadrontherapy were adapted so that the Geant4 output data were integrated into the SISCODES eye model for the generation of isodose curves. The simulation results were integrated with the voxels model of the ocular region on the SISCODES, where the dose spatial distribution is reproduced on an isodose curve. The SISCODES computer system belongs to the Nucleus of Ionizing Radiations research group of the Post Graduation Course in Nuclear Sciences and Techniques of Universidade Federal de Minas Gerais (UFMG), Belo Horizonte, MG, Brazil(6). Eye phantom An ocular phantom previously described by Mourão & Campos(7) was utilized in the present simulation. The voxel model of the ocular region is composed by 82 × 100 × 43 voxels. This is a non-isotropic model which corresponds to a 41 × 50 × 38.7 mm3 volume, representing a matrix whose volume element's dimensions are 0.5 × 0.5 × 0.9 mm3, according to Mourão & Campos(7). Such model originates from the Visible Man Project (VMP)(8) , and was adapted and imported into the SISCODES which comprises modules for the creation of voxel/volume models based on a sequence of section images, and images processing. The model was converted into a gray-scale image matrix by the SISCODES images processing module(7). The SISCODES' three-dimensional eye model was composed by voxels by means of the overlapping of 43 transverse sections of the VMP(7) model. In such a model, the main structures of the ocular region such as the eyeball, muscles and optic nerve are represented. The material considered for the proton transport in the phantom was water; but the isodoses are plotted in overlapping with the material present in the eye phantom. Definition of energy modulator, absorbing material and collimator In clinical applications, several accessories are utilized to form or positioning the proton beam for each treatment and, before the treatment, such accessories must be properly configured and checked by the planning system(9). In the Geant4 hadrontherapy application, devices are placed along the proton beam trajectory to adapt the irradiation to the shape and to the distance from the tumor, and to protect adjacent healthy tissues, as follows: - The absorbing material (range shifter) to degrade the energy from the primary beam to a determined value, consequently defining its maximum range. - The modulator or beam modulation system for energy scattering so as to comprise the whole tumor. The modulator, made from polymethylmethacrylate (PMMA), ensures the homogeneity of depth-dose distribution, comprising the entire target volume, spreading the beam with the determined modulation and generating the curve denominated spread-out Bragg peak (SOBP). The SOBP is generated by the rotation of a device (ring) with propeller-like blades of different thicknesses. Such device spins around an axis parallel to the proton beam, and such spinning motion in degrees can be redefined at each simulation performance(10). - The collimator defines the shape and diameter of the proton entry beam, laterally molding the form of the energy deposition. Variations in the collimators configuration were applied in the experiments performed. Proton beam energy - The irradiation dose is delivered at a given depth that depends upon the incident protons energy. The simulations were performed by configuring the energy of the primary proton particles beam at 62.0 MeV, whose range is approximately three centimeters(10). Evaluation of the pure and modulated Bragg peak The absorbed dose curve as a function of the absorbing material thickness shows a typical Bragg peak, whose width depends on the nature of the radiation, the scattered energy (straggling) and the absorbing material itself. The initial segment of the dose versus depth curve, before the Bragg peak, presents a practically constant dose distribution representing approximately 30% of the maximum dose. The pure Bragg peak is very narrow when reaching the target volume. Its occurrence in depth is dependent on the beam energy, i.e., if the energy is increased the Bragg peak occurs more deeply in the tissue. For the irradiation of target volumes one can perform the controlled variation of the proton entry energy by producing overlap of multiple Bragg peaks. Also, the incidence of a monoenergetic proton beam may increase the thickness of the absorbing material superimposed to the beam entry. Such overlapping may also be reproduced by the rotation of a propeller-like device with continuously varying blade thickness, whose rotation direction defines the desired thickness. Thus, a modulated dose spectrum (SOBP curve) is also generated, with an amplified Bragg peak formed by the overlap of several dose profiles, representative of varied absorber thicknesses, covering the whole width of the target volume. The pure Bragg peak (without modulation) will be produced for the incident proton energy. Modulated Bragg peaks will also be produced as a function of the specific thicknesses of the absorbing material, or range shifter. Evaluation of depth absorbed dose and dose rate spatial profiles The results from simulations with Geant4 were reproduced in depth-dose distribution curves. Additionally, the Geant4 code generates data on the energy delivered on the X, Y and Z voxels coordinates that were transferred to SISCODES. The conversion of the Geant4 output data into SISCODES is performed by a specific code that makes the interface between the two environments, besides calculating the delivered dose rate in each voxel by means of the ratio delivered energy in MeV/specific mass of each voxel converted into Gy/p (Gray/incident proton). The total absorbed dose rate corresponds to the product of the dose by incident particle unit by the applied proton current, and the absorbed dose corresponds to the product of the dose rate by the exposure time. The absorbed dose calculated at the phantom isocenter depends upon the combination of the modulation system and the range shifter thickness. The dose released by the proton beam is deposited in a voxel geometry of any material and shape that can then be converted for the SISCODES system. For the treatment of uveal melanoma the total applied dose is approximately 60 Gray equivalent (GyE), delivered in four 15 Gy fractions(10). RESULTS Generation of pure and modulated Bragg peak Figures 1 and 2 represent the Bragg peak and the modulated Bragg peak (SOBP curve) without and with beam modulation, applying a collimator with a final diameter of 7.5 mm, and without absorbing material, with incidences of 1.00 × 106 protons and 1.08 × 106 protons, respectively. For the production of the SOBP, the PMMA ring was rotated with the resolution of one degree (1º), completing 360º in total, utilizing 3.00 × 103 protons at each step, according to recommendation(9) to generate a representative dose. 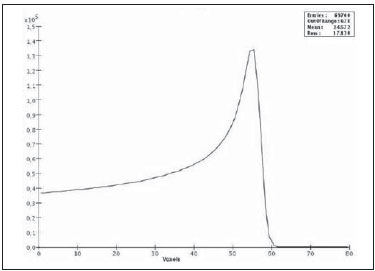 Figure 1. Bragg peak produced without modulation, processing time of 20 hours and 22 minutes. 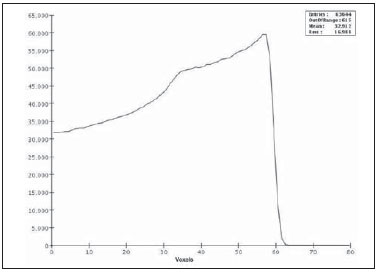 Figure 2. SOBP curve with 360° rotation, processing time of 19 hours and 38 minutes. Table 1 presents the simulations results, producing Bragg peak and SOBP, with and without modulation, processing 1.00 × 106 protons and 1.08 × 106 protons, respectively. The collimator diameter and absorbing material thickness (range shifter) parameters were entered in different configurations. Dose spatial distribution in the eye model Figures 3 to 6 represent the isodose curves in the eye model generated at the SISCODES, considering changes in the positioning of the proton beam entry window. The dose rates are normalized as a function of maximum dose for a beam entry point, number of incident protons, specified modulator and absorber. 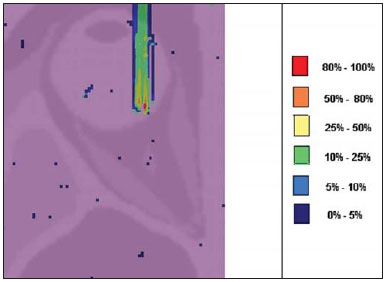 Figure 3. Isodose curve, superimposed on the eye model, generated in the SISCODES, corresponding to the simulation performed without modulation, final collimator diameter of 1 mm and 8 mm of absorbing material. Distance of 5 mm at right from eye lens center. 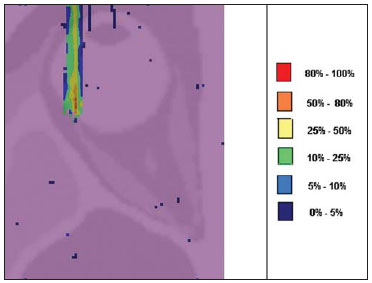 Figure 4. Isodose curve, superimposed on the eye model, generated in the SISCODES, corresponding to the simulation performed without modulation, final collimator diameter of 1 mm and 8 mm of absorbing material. Distance of 8 mm at the right from eye lens center. 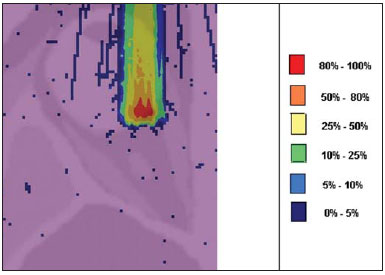 Figure 5. Isodose curve, superimposed on the eye model, generated in the SISCODES, corresponding to the simulation performed without modulation, final collimator diameter of 3.5 mm and 6 mm of absorbing material. Displacement of 5 to 7 mm at the right from the eye lens center. 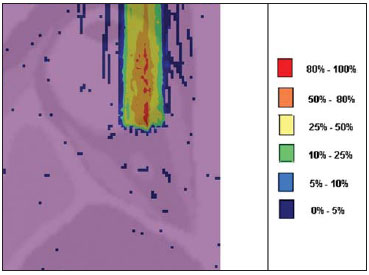 Figure 6. Isodose curve, superimposed to the eye model, generated in the SISCODES, referent to the simulation executed with modulation, rotation of 360°, final collimator diameter of 3.5 mm and 6 mm of absorbing material. Displacement of 5 to 7 mm at right from the eye lens center. Simulations without modulation were performed, with incident beams of 1.00 × 106 protons, resulting in a maximum deposited dose of 2.69 × 10-10 Gy/proton and 2.49 × 10-10 Gy/proton, in processing times of 18 hours and 15 minutes and 17 hours and 54 minutes, respectively. These results are presented on Figures 3 and 4. Figure 5 presents a situation without modulation, with 3.00 × 106 protons, which resulted in a maximum deposited dose of 3.80 × 10-10 Gy/proton, in a processing time of 52 hours and 19 minutes. The simulation with modulation and 3.24 × 106 incident protons (Figure 6), resulted in a maximum deposited dose of 2.06 × 10-10 Gy/proton, in a processing time of 61 hours and 14 minutes. The simulations were performed by directing the entry beam at points in different ocular quadrants represented in the voxel model so as to comprise the targets to be irradiated in different positions. Figures 5 and 6 represent simulations performed with a three times higher number of protons than previous simulations, with the purpose of improving the deposited dose accuracy. Additionally, during the simulation, the beam was moved by 5 to 7 mm to the right from the eye lens center, maintaining the same final collimator diameter and absorbing material thickness for beam incidences with and without modulation. DISCUSSION In the curves resulting from simulations without and with beam modulation represented on Figures 1 and 2, one observes that the range of both curves was 30 mm, with the unit in the X axis corresponding to voxels, whose thickness is 0.5 mm. The SOBP curve apex on Figure 2 comprised an extent of approximately 14 mm when the modulation system was utilized, demonstrating that the use of the modulator amplifies the dose distribution extent in the beam direction. In clinical applications, the beam modulation is utilized to cover the neoplastic target or the region of interest with greater dose deposition. For the simulations represented on Figures 1 and 2, the maximum dose expressed in Gray/proton were calculated at 3.04 × 10-9 and 1.38 × 10-9, respectively. In order to achieve the clinical dose for treatment of ocular melanoma (60 Gray - 4 sessions of 15 Gray), considering the mentioned simulations conditions, incidences of 6.17 × 109 protons and 13.59 × 109 protons, with a current intensity of 0.992 nA and 2.188 nA, respectively, would be necessary at each treatment session. Based on the simulations data represented by the values on Table 1, the following comments should be taken into consideration: - One observes that the SOBP curves generated from the modulated beam achieve a greater depth and homogeneity in the dose distribution, in relation to the Bragg peak without modulation. For clinical treatments, multiple modulated beams are utilized to comprise the entire tumor volume, in a scanning process with translational motion of the beam entry point, with changes of the absorber. - The range of the beam decreases as the absorbing material thickness increases. Table 1 presents the effect of different absorbing material thicknesses on the dose depth. - Both the increase in the absorbing material thickness and the reduction in the collimator diameter reduce the deposited dose value, requiring the increase in incident protons and/or applied current. However, the configurations of such parameters are of fundamental importance for the personalization of the radiotherapy protocol in order to appropriately address the specific characteristics of the target to be irradiated. - The reduction of the collimator diameter by 50% (ID 3, 4, 8 and 9) resulted in dose reduction of 41% and 36%, with beams without and with modulation, respectively. The target volume dimensions are taken into consideration in the collimator configurations. - A dose reduction of respectively 74% and 46% for the beam without and with modulation was observed with the non application of absorbing material and utilizing it with 4 mm (ID 1, 2, 5 and 7). The beam range was reduced by 5 mm, approximately 17% in relation to the beam without absorbing material. By applying 0 mm, 2 mm and 4 mm of absorbing material (ID 5, 6 and 7), with a modulated beam, the dose reduction was 20% and 33% for each interval. The calculations of necessary conditions to achieve the clinical dose of 60 Gray (4 sessions of 15 Gray) utilized for the treatment of ocular melanoma were based on data presented on Table 1. Such parameters are the number of protons to be utilized for irradiation and the current (nA) necessary for producing the mentioned dose, presented on Table 2. The relevant data for clinical application are those regarding to the modulated beam, in which one observes that the lowest value of the applied current corresponds to approximately 10% of the highest value, in relation to the application of the absorbing material. Additionally, the current values are compatible with actual values utilized in accelerators in operation(1). The time unit established on Table 2 is the second. Thus, in the presented simulations, a specific set of voxels whose volume receives the absorbed dose > 80% is exposed for a unitary period of one second. Such volume covered by a single incidence is herein defined as a sector. Then, the tumor volume should be covered with pre-established absorbed doses with the irradiation of multiple sectors. In this case, several incidences are necessary and thus the treatment time must be prescribed on the basis of the tumor geometry. As regards protons extraction, the ICRU 59(13) defines that proton-therapy accelerators must extract more than 5.00 × 1010 protons per second. Considering that the session time in ocular radiotherapy ranges from 30 to 60 seconds(11), the values for incident protons presented on Table 2 are viable when applied in multiple fractions covering the tumor as required by the clinical application. Considering the results represented by Figures 3 to 6, in order to achieve the clinical dose for treatment of ocular melanoma (60 Gray - 4 sessions of 15 Gray), incidences of 6.98 × 1010 protons, 7.54 × 1010 protons, 4.93 × 1010 protons and 9.09 × 109 protons, with current intensities of 11.23 nA, 12.13 nA, 7.93 nA and 14.63 nA, respectively, will be required at each tumor sector. A computer with a Pentium Dual Core 2 GHz processor was utilized in for the simulations described in the present study. In the case of clinical applications, the use of several parallel processors, as in a computer cluster, is recommended for the reduction of processing time, in a viable manner for the mentioned application. CONCLUSION Relevant aspects related to the planning system for proton radiation therapy, such as the absorbing material, modulation, tumor size, collimator dimensions, incident proton energy, proton current and isodose curves generation to assure a homogeneous coverage of the tumor, were evaluated and configured according to the characteristics of the target volume to be treated. The simulation results demonstrated typical isodoses produced in protocols for ocular proton radiation therapy. Such results reinforce the need for the development of applications in the field of proton radiation therapy planning. The integration of different computational environments such as Geant4 and respective application libraries with the SISCODES extends the conditions for planning proton radiation therapy, adding new functionalities that will contribute for future studies approaching radiotherapy simulations. REFERENCES 1. PTCOG: Particle Therapy Co-Operative Group. [acessado em 27 de março de 2010]. Disponível em: http://ptcog.web.psi.ch 2. Thornton AF, Fitzek M, Klein S, et al. Proton beam radiotherapy: a specialized treatment alternative. Commun Oncol. 2007;4:599-607. 3. Virgili G, Gatta G, Cicolallo L, et al. Incidence of uveal melanoma in Europe. Ophthalmology. 2007;114:2309-15. 4. Singh AD, Topham A. Incidence of uveal melanoma in the United States: 1973-1997. Ophthalmology. 2003;110:956-61. 5. Cirrone GAP, Cuttone G, Di Rosa F, et al. The Hadrontherapy Geant4 advanced example. In: 4th Workshop on Geant4 Bio-medical Developments, Geant4 Physics Validation; 2005 July 13-20; Genova, Italy. 6. Trindade BM. Desenvolvimento de sistema computacional para dosimetria em radioterapia por nêutrons e fótons baseado em método estocástico SISCODES [tese de mestrado]. Belo Horizonte, MG: Universidade Federal de Minas Gerais; 2004. 7. Mourão AP, Campos TPR. Development of a human eye model for ophthalmic brachytherapy dosimetry in heterogeneous medium at the uvea. In: Biomat 2007 - International Symposium on Mathematical and Computational Biology; 2007 Nov 24-29; Armação dos Búzios, RJ, Brasil. 8. National Library of Medicine. The visible human project. [acessado em 30 de março de 2010]. Disponível em: http://www.nlm.nih.gov/research/visible/visible_human.html 9. Slater JM, Miller DV, Slatter JW. Developing a clinical proton accelerator facility: consortium-assisted technology transfer. In: Particle Accelerator Conference, Accelerator Science and Technology., Conference Record of the 1991 IEEE; 1991 May 6-9; San Francisco, CA, USA. 10. Cirrone GAP, Cuttone G, Di Rosa F, et al. Monte Carlo based implementation of an energy modulation system for proton therapy. In: Nuclear Science Symposium Conference Record, 2004 IEEE; 2004 Oct 16-22. 11. International Commission on Radiation Units and Measurements. Clinical proton dosimetry part I: Beam production, beam delivery and measurement of absorbed dose. ICRU Report 59. Bethesda, MD: ICRU; 1998. 1. Fellow PhD degree, Department of Nuclear Engineering, Universidade Federal de Minas Gerais (UFMG), Technologist at Centro de Desenvolvimento da Tecnologia Nuclear/Comissão Nacional de Energia Nuclear (CDTN/CNEN), Belo Horizonte, MG, Brazil 2. PhD, Professor at Department of Nuclear Engineering, Universidade Federal de Minas Gerais (UFMG), Belo Horizonte, MG, Brazil Study developed at the Department of Nuclear Engineering of Universidade Federal de Minas Gerais (UFMG), Belo Horizonte, MG, Brazil Mailing address: Marília Tavares Christóvão Universidade Federal de Minas Gerais (UFMG), Departamento de Engenharia Nuclear Cidade Universitária da UFMG, Pampulha 31270-901. Belo Horizonte, MG, Brazil E-mail: marilia@cdtn.br Received April 15, 2010 Accepted after revision June 11, 2010 |
|
Av. Paulista, 37 - 7° andar - Conj. 71 - CEP 01311-902 - São Paulo - SP - Brazil - Phone: (11) 3372-4544 - Fax: (11) 3372-4554