Radiologia Brasileira - Publicação Científica Oficial do Colégio Brasileiro de Radiologia
AMB - Associação Médica Brasileira CNA - Comissão Nacional de Acreditação
 Vol. 40 nº 6 - Nov. / Dec. of 2007
Vol. 40 nº 6 - Nov. / Dec. of 2007
|
ORIGINAL ARTICLE
|
|
Proposal of a methodology for individualized iodine-131 therapy for Graves' disease in patients with hyperthyroidism |
|
|
Autho(rs): Francisco de Araujo, Rossana Corbo de Melo, Ana Maria de Oliveira Rebelo, Bernardo Maranhão Dantas, Ana Letícia A. Dantas, Eder Augusto de Lucena |
|
|
Keywords: Graves' disease, Hyperthyroidism, Iodine therapy, Iodine-131, Scintillation gamma camera, Uptake probe |
|
|
Abstract:
IMaster in Radioprotection and Dosimetry, Instituto de Radioproteção e Dosimetria/Conselho Nacional de Energia Nuclear (IRD/CNEN), Rio de Janeiro, RJ, Brazil, Researcher, Conselho Nacional de Desenvolvimento Científico e Tecnológico - CNPq (PCI Scholarship)
INTRODUCTION The first studies about the thyroid function have been developed with 131I, that up to the present time has been utilized in nuclear medicine for therapy of hyperthyroidism and, particularly in cases of thyroid ablation for treatment of cancer. Therapeutic 131I doses are orally administered in the form of liquid or capsules(1). Radiodine advantages include: easy administration, effectiveness, low cost and absence of pain. When a sodium iodine solution is orally administered, the iodine is rapidly absorbed and concentrated in the thyroid where it is incorporated into storage follicles, with a five-day effective half-life. According to UNSCEAR data(2), 90% of therapeutic procedures in nuclear medicine utilize 131I. The therapeutic doses present an activity ranging from 100 MBq (2.7 mCi) to 1000 MBq (27 mCi) for treatment of hyperthyroidism, and 4 GBq (108 mCi) to 8 GBq (216 mCi) for treatment of thyroid cancer(3). The activity uptake by the thyroid gland after 131I administration varies among patients, depending on several factors such as: degree of iodine uptake, mass of uptaking tissue, effective iodine half-life in the thyroid, distribution of radioactivity throughout the tissue, and cells radiosensitivity. However, scarce conclusive information is available in the literature regarding the patients absorbed dose. As a function of the magnitude of the administered activity for treatment with radioiodine, the main risks for patients involve increase in the probability of developing cancer in different organs or tissues, and the effects on the descendants of women at childbearing age(4). The realistic evaluation of these risks requires a biokinetic analysis of the 131I behavior in the organism, followed by the calculation of the patients absorbed dose. A consensus is still to be achieved on the ideal protocol for treating hyperthyroidism. Frequent discussions have been held on which protocol would contribute with the best clinical results. Currently, different protocols are utilized to define the activity to be administered in the radioiodine therapy for hyperthyroidism, but not all of them take the thyroid absorbed dose into consideration. Some protocols utilize a fixed administered activity (standard), without considering biokinetic parameters such as the thyroid gland volume, the iodine effective half-life in the thyroid, and the individual uptake(5). When a 10 mCi standard activity is utilized in a patient affected by Graves' disease, the thyroid absorbed-dose may range from 60 Gy to 600 Gy(5). In patients with a short iodine half-life, the absorbed dose per activity unit will be low and, possibly, the treatment will not succeed, requiring a second therapy. For high doses in patients with long effective half-life, probably there will be an unnecessary exposure(5). Among those who defend the absorbed-dose calculation, quite a few take only some parameters into consideration: thyroid gland volume, only thyroid uptake, or only thyroid mass. They say that the calculation of the effective half-life would require several visits of the patient to the hospital before the treatment, increasing the cost and resulting in a long wait for starting the therapy (5). The most frequent causes for hyperthyroidism are diffuse toxic goiter, also know as Graves' disease, multinodular toxic goiter, and toxic adenoma. Graves' disease is the most frequent one (80%)(6). The effective iodine half-life in the thyroid of patients affected by Graves' disease is low, while the uptake is high as compared with the multinodular and uninodular goiters(5). The typical thyroid iodine retention curves show that the highest level of iodine uptake in patients affected by diffuse toxic goiter occurs, on average, 12 hours after the radionuclide administration. This characteristic is extremely relevant in the calculation of the effective half-life in the interval between 14 and 30 hours following the radioiodine administration to the patients. The utilization of the available tools and routine procedures in the division of nuclear medicine has made this low cost methodology feasible, simple and effective.
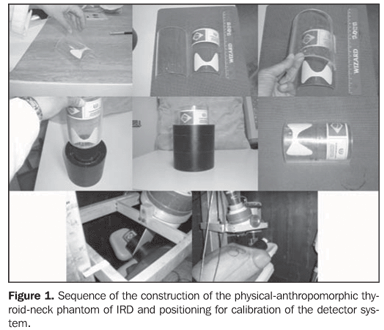
MATERIALS AND METHODS Production of the thyroid phantoms The experimental part of this study was initially developed in the Laboratory of In Vivo Measurements of Instituto de Radioproteção e Dosimetria (IRD), where the thyroid phantom was constructed with a 100 mm-diameter filter paper (Whatman) trimmed in a thyroid shape and size(7). This phantom was impregnated with a mass consisting of 241.86 mg of 131I solution, with specific activity of 3.075 MBq/g (83.1 µCi/g) and 1.1% total uncertainty. This solution was previously calibrated by the National Laboratory of Ionizing Radiation Metrology (Laboratório Nacional de Metrologia das Radiações Ionizantes) of Instituto de Radioproteção e Dosimetria da Comissão Nacional de Energia Nuclear (IRD-CNEN). So, the 131I activity added to the filter paper on the calibration date was 743646.94 Bq (20.1 µCi), a value compatible with the administered diagnostic activity for 131I uptake tests (routine procedure) in the patients referred for radioiodine therapy for hyperthyroidism. The thyroid phantom utilized in the present study was based on a previously available model (Figure 1).
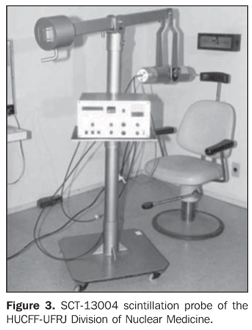
Optimization and calibration of in vivo 131I detection systems This phase was developed in the Division of Nuclear Medicine of Hospital Universitário Clementino Fraga Filho da Universidade Federal do Rio de Janeiro (HUCFF-UFRJ), and consisted in the calibration of the Siemens Diacam Gamma camera equipped with a NaI(Tl) crystal, with 2" × 2" and 59 photomultiplier thicknesses, pinhole-type lead collimator (Figure 2), and a SCT-13004 scintillation probe (Figure 3). This priority of this methodology is the optimization of the in vivo detection system, for calculating the efficiency of 131I detection in the thyroid.
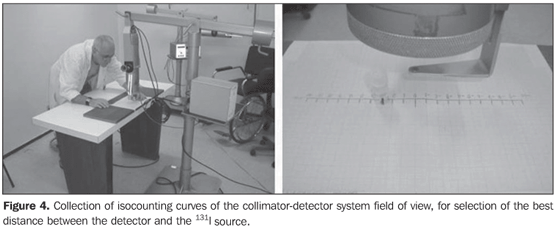
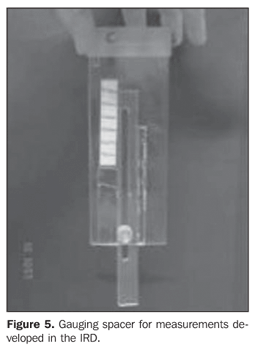
Collimator-detector assembly field of view For establishing the best working distance between the source and the detector, curves typical of the collimator-detector assembly field of view regarding the equipment involved in this experiment were traced. The method utilizes isocounting or isoresponse curves for each of the systems(9). These curves were obtained by the positioning of the detector at different distances from a punctiform 131I source, with calibration energy set at 364 keV. So, this source is placed on a table covered with a millimetred paper sheet, on the position "zero" coinciding with the central axis of the collimator. For each of the distances, the punctiform source is moved in 1.0 cm steps, from the point "zero" up to 10.0 cm at right and at left, perpendicularly to the central axis of the collimator (Figure 4). For each source position, three 30-second measurements were performed. The counting rate corresponded to the average of these three measurements. For evaluating the field of view of the pinhole collimator-detector assembly, without the reducing ring, five different distances (42.8 cm, 43.8 cm, 44.8 cm, 45.8 cm and 46.8cm) were measured with the aid of a gauging spacer developed at IRD (Figure 5). For the SCT-13004 detector-collimator assembly three different distances (20 cm, 25 cm and 30 cm) were measured with the ruler coupled with the assembly (Figure 4). The function of the ruler and "spacer" is to keep the accuracy and reproducibility of the measurements.
Calibration factor for gamma camera and scintillation probe Initially, the thyroid-neck phantom was positioned for counting the background radiation with the thyroid phantom free from contamination, and later with the phantom impregnated with the 131I solution, as previously described. In both cases, the detector was positioned at 42.8 cm, 43.8 cm, 44.8 cm, 45.8 cm and 46.8 cm from the phantom, the countings being performed in five minutes for each distance. Each measurement was repeated for three times for a better statistical result. The same procedure was adopted for obtention of the calibration factor of the scintillation probe, only with a variation of the distances between the detector and the phantom (20 cm, 25 cm and 30 cm). Determination of biokinetic data The mathematic simulation of data from the literature(5,10) regarding the iodine retention curve based on measurements in the thyroid of patients with Graves' disease has allowed the evaluation of convenient time intervals for calculating of activities values for determination of the radioiodine effective half-life and the initial uptake in the thyroid gland. The relation between the thyroid absorbed dose and administered activity required for the therapy was calculated by means of an equation developed by Marinelli-Quimby(11). D/A = 0.043 Uo Tef/V where: D/A is the absorbed-dose/unit of administered activity (Gy/MBq); Uo is the initial uptake extrapolated for zero time (%); Tef is the effective half-life (day); V is the estimated thyroid volume (cm3). The thyroid density was assumed to be 1g/cm3. A Microsoft Excel worksheet was created for an appropriate calculation of values related to the equation variables.
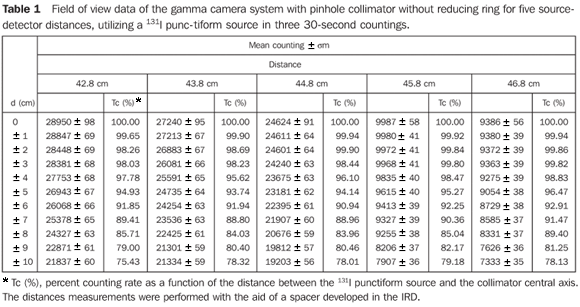
RESULTS Determination of the field of view curves of the collimator-detector system These curves allow the determination of the collimator-detector system field of view for the equipment involved in the present experiments, indicating the best source-detector distance. The most informative method for demonstrating these characteristics for each device is based on the isocounting or isoresponse curves. In this region named "field of view", the values from any region of the thyroid gland are accounted with an uniform sensitivity, besides reducing not only the environment background radiation, but also the background radiation coming from other regions of the patient's body. Table 1 demonstrates mean values of three countings for each source position in relation to the collimator central axis, and percent values of counting rates regarding the radioactive source at a maximum counting position with the respective associated uncertainties.
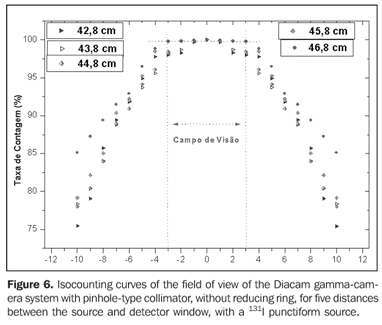
Figure 6 presents the isocounting curve of the pinhole lead collimator without the reducing ring, where the percent counting rate is shown as a function of the distance between the 131I punctiform source and the central axis of the collimator.
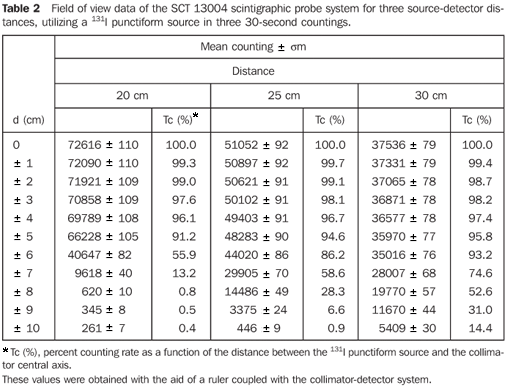
Table 2 demonstrates the mean values of three countings for each position of the source in relation to the central axis of the collimator, and the percent counting rates regarding the radioactive source in the position of maximum counting with the respective associated uncertainties.
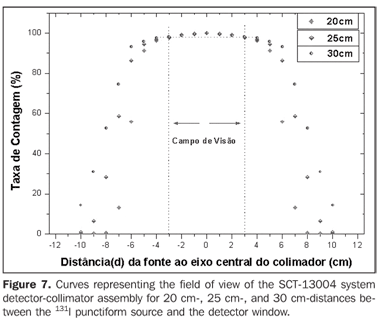
Figure 7 presents the isocounting curve of the SCT-13004 system detector-collimator for distances of 20 cm, 25 cm and 30 cm, where the percent counting rate is shown as a function of the distance between the 131I punctiform source and the central axis of the collimator.
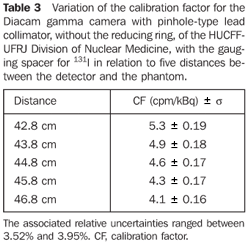
Optimization and determination of the calibration factor For the Diacam gamma camera system with a pinhole lead collimator, without reducing ring - Table 3 shows the calibration factors for 131I and uncertainties associated with the respective distance between the detector and the phantom. For the Siemens Diacam gamma camera system, the experiments were developed with a pinhole-type lead collimator without the reducing ring and with a gauging spacer.
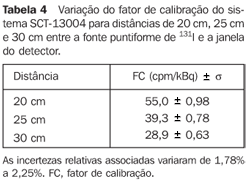
For the scintigraphic probe SCT-13004 - Table 4 presents a variation of the calibration factor of the SCT-13004 system for 20 cm-, 25 cm-, and 30 cm-distances between the 131I punctiform source and the detector window.
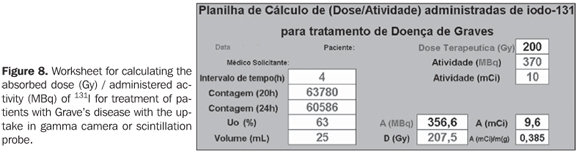
Calculation of biokinetic data Data on the worksheet (Figure 8) were worked out with resources from mathematics and physics, for obtention of biokinetic parameters.
In the measurements with gamma-camera or scintillation probe for 131I uptake (test activity) by the thyroid of patients affected by Graves' disease, the counting rates (cpm) are collected through two successive tests in a time interval (t), and the initial activity (A0) and final activity (A) are calculated for the considered time interval, utilizing the values for calibration factor (CF) obtained in the present study (equation 1). These values will be applied to the equation (2), for obtention of lef (decay constant), and successively to the equation (3), for calculating the effective half-life in the organ (Tef). The initial uptake (U0) will be calculated by extrapolation with the values of the previously mentioned uptake tests. The thyroid gland volume can be estimated by palpation or ultrasonography. These data and the corresponding therapeutic activity will be conveniently recorded in a Microsoft Excel worksheet for calculation of the desired absorbed-dose or vice-versa, as shown on the worksheet in Figure 8.
DISCUSSION Figure 6 shows the curves with a small plane segment, where the system will present as a result the same number of countings. This plane segment increases in diameter as the distance between the source and the detector window increases. The field of view is represented by the segment between the vertical dotted lines, and its size is indicated on the abscissas axis. For the evaluation of the thyroid radioiodine uptake with the Diacam gamma camera system, the source-detector distances of 45.8 cm and 46.8 cm where those that indicated a better radiation counting response, presenting a field of view compatible with the thyroid gland size of approximately six centimeters (abscissas -3 and +3) for patients affected by Graves' disease. The distances of 42.8 cm, 43.8 cm and 44.8 cm, although with countings number higher than the distances of 45.8 cm and 46.8 cm, but with a field of view < 6 cm, the system will not record all of the radiations from the thyroid gland, as it can be observed on the isocounting curves. For distances above 46,8 cm, the detector system will record, besides the radiation from the thyroid gland, a significant quantity of the environment background radiation, and those from other regions of the patient's body. Between distances of 45.8 cm and 46.8 cm, the first one is preferred for presenting a higher number of countings considering the proximity of the source and, as a result, a lower associated uncertainty. The calibration factor of 4.3 ± 0.17 (cpm/kBq) utilized in the present study is associated with this distance. Notwithstanding, the calibration factor value corresponding to the 46.8 cm distance also can be utilized in the calculation of the absorbed-dose (Gy)/administered activity (MBq) ratio, for the patients submitted to radioiodine therapy for hyperthyroidism. These calibration factor will be a function of the thyroid gland enlargement (with a field of view > 6 cm), depending on the disease severity. On the isocounting curves in Figure 7, one can observe that the best responses for the SCT-13004 system are those in the 25 cm and 30 cm. In this case, the 25 cm distance is preferred for, besides providing a higher number of countings and, consequently a low associated uncertainty, is the most utilized in the measurements of thyroid radioiodine uptake, in routine procedures of nuclear medicine. In this distance, it can be observed that the procedure is more comfortable for the patients, the system sensitivity is appropriate for the measurements in relation to the 30 cm distance, besides presenting a field of view compatible with the thyroid size in patients with Graves' disease. The calibration factor observed for this distance was 39.3 ± 0,78 (cpm/kBq). The thyroid-neck phantom as well as the calibration protocol developed have shown to be appropriate for the purposes of the present study. Both the gamma camera and the uptake probe may be utilized for determining the 131I activity in the patients' thyroid. This procedure will be later applied for optimization of the individualized activity to be administered to each patient. This methodology is feasible and low-cost, considering that the patients will visit the hospital for only two times, the first visit for receiving the test-activity, and the second one for being submitted to the uptake processes (%) between the14-hour and the 30-hour time intervals, where a data collection will be performed including counting rates required for calculating biokinetic data, (effective iodine half-life in the thyroid and initial uptake), and immediately after, the calculated activity administration. The methodology is effective and reliable because utilizes all of the biokinetic parameters required for calculation of the thyroid absorbed-dose (therapeutic dose). Acknowledgements The authors thank Instituto de Radioproteção e Dosimetria da Comissão Nacional de Energia Nuclear (IRD/CNEN) and Hospital Universitário Clementino Fraga Filho da Universidade Federal do Rio de Janeiro (HUCFF-UFRJ), for the technical support. This research was evaluated and approved by the HUCFF-UFRJ Committee for Ethics in Research, on May 15, 2006.
REFERENCES 1. Thompson MA. Radiation safety precautions in the management of the hospitalized 131I therapy patient. J Nucl Med Technol 2001;29:61–66. [ ] 2. United Nations Scientific Committee on the Effects of Atomic Radiation. UNSCEAR 2000 Report to the General Assembly, with scientific annexes. Volume I: Sources. Vienna, Austria: UNSCEAR, 2000. [ ] 3. International Commission on Radiological Protection. Release of patients after therapy with unsealed radionuclides. ICRP Publication 94. Oxford: Pergamon Press, 2004. [ ] 4. Brandão CDG, Antonucci J, Correa ND, Corbo R, Vaisman M. Efeitos da radioterapia nas gerações futuras de mulheres com carcinoma diferenciado de tireóide. Radiol Bras 2004;37:51–55. [ ] 5. Jönsson H. Radioiodine therapy of hyperthyroidism. Simplified patient-specific absorbed dose planning. Malmö: Department of Radiation Physics, Lund University, 2003. [ ] 6. Nyström E, Berg G, Jansson S, et al. Thyreotoxikos hos vuxna. Klippan, Sweden: Ljungbergs Tryckeri AB, 1999. [ ] 7. International Commission on Radiological Protection. General principles for the radiation protection of workers. ICRP Publication 75. Oxford: Pergamon Press, 1997. [ ] 8. Dantas BM. Bases para a calibração de corpo inteiro utilizando simuladores físicos antropomórficos. (Tese de Doutorado). Rio de Janeiro, RJ: Universidade do Estado do Rio de Janeiro, 1998. [ ] 9. Silva CB. Medida da captação de iodo pela tireóide: análise comparativa entre sistema gama-câmara com colimador tipo "pinhole" e sistema 13S002. (Dissertação de Mestrado). Rio de Janeiro, RJ: Instituto Militar de Engenharia, 2001. [ ] 10. Williams RH. Textbook of endocrinology. 4th ed. Philadelphia-London-Toronto: WB Saunders, 1968. [ ] 11. Marinelli LD, Quimby EH, Hine GJ. Dosage determination with radioactive isotopes II. Practical considerations in therapy and protection. Am J Roentgenol Radium Ther 1948;2:260–281. [ ]
Received January 25, 2007. Accepted after revision May 7, 2007.
* Study developed at Instituto de Radioproteção e Dosimetria/Conselho Nacional de Energia Nuclear (IRD/CNEN), Rio de Janeiro, RJ, Brazil. |
|
Av. Paulista, 37 - 7° andar - Conj. 71 - CEP 01311-902 - São Paulo - SP - Brazil - Phone: (11) 3372-4544 - Fax: (11) 3372-4554