Radiologia Brasileira - Publicação Científica Oficial do Colégio Brasileiro de Radiologia
AMB - Associação Médica Brasileira CNA - Comissão Nacional de Acreditação
 Vol. 40 nº 6 - Nov. / Dec. of 2007
Vol. 40 nº 6 - Nov. / Dec. of 2007
|
ORIGINAL ARTICLE
|
|
Reproducibility in the assessment of acute pancreatitis with computed tomography |
|
|
Autho(rs): Edison de Oliveira Freire Filho, David Carlos Shigueoka, Daniel Bekhor, Renata La Rocca Vieira, André Fukunishi Yamada, Maxime Figueiredo de Oliveira Freire, Sergio Ajzen, Giuseppe D'Ippolito |
|
|
Keywords: Pancreatitis, X-ray computed tomography, Disease severity index, Prognosis, Contrast media, Necrosis |
|
|
Abstract:
IFellow PhD degree, Department of Imaging Diagnosis, Universidade Federal de São Paulo/Escola Paulista de Medicina (Unifesp/EPM), São Paulo, SP, Brazil
INTRODUCTION Acute pancreatitis is a relatively common disease whose presentation may range from mild, self-limited to a sudden-onset disease resulting in death within few days. In the last decades, the difficulty in a reliable prediction of the progression of the disease has led to the search for objective methods to evaluate its severity(1). Once the acute pancreatitis diagnosis is established, the intensity, duration and type of treatment will depend on an early definition of the disease severity. Such definition that is based on objective parameters is essential for the early prediction of risks for clinical complications and identification of potentially fatal presentations(2-4). Up to the seventies, the disease severity evaluation was performed in a subjective way, mainly by the presence or absence of several clinical parameters such as tachycardia, fever, dyspnea, oliguria, paralytic ileus, etc.(3). Multiple systems based on clinical-laboratory parameters have been devised to assess the severity of acute pancreatitis. These include Ranson criteria, original and modified Glasgow criteria, simplified acute physiology (SAP) score, and acute physiology and chronic health evaluation II (APACHE II) score(1-3,5-7). Difficulty and complexity of calculation constitute the major limitations of these systems, considering their dependence on numerous clinical and laboratory variables. In 1985, Balthazar et al.(3) developed a system for assessing the severity of acute pancreatitis based on peripancreatic findings and alterations in the pancreatic morphology observed on computed tomography (CT), and that, in the present study, is denominated morphological index. In 1990(8), these same authors demonstrated, by means of contrast-enhanced computed tomography, the correlation between the pancreatic necrosis extent and acute pancreatitis prognosis. Based on an association between the criteria described in 1985 and the pancreatic necrosis assessment, the authors created the severity index for acute pancreatitis. The parameters considered in the calculation of the morphological index and CT severity index are subjective and therefore are susceptible to interobserver variations or even variation by a same observer at different moments (intraobserver variations), so they may present different values depending on the observer, his/her experience, and the moment of the analysis. However, it is important to define the method reproducibility in order to add value to the results obtained, transmitting confidence in the conduction of the treatment and evolutive follow-up. Despite the widespread utilization of these indices both in the literature and clinical practice, few studies have evaluated their reproducibility(911) and there is no study analyzing possible intraobserver disagreements. Although in the opinion of several authors, unenhanced CT can demonstrate pancreatic and peripancreatic inflammatory alterations, being sufficient to define the prognosis for acute pancreatitis(2,7,12-14), up to the present moment there is no study evaluating if unenhanced CT can reproduce contrast-enhanced CT findings for an accurate morphological index calculation in cases of acute pancreatitis. The present study was aimed at measuring the reproducibility of unenhanced and contrast-enhanced CT in the evaluation of the disease severity in patients with acute pancreatitis.
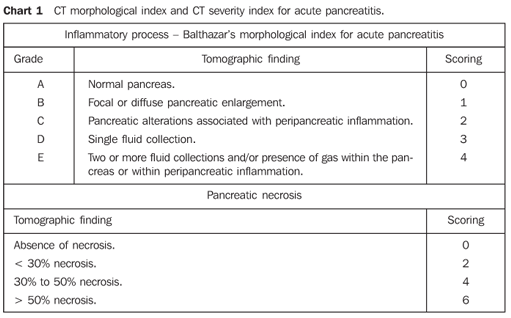
MATERIALS AND METHODS In the period between June/2003 and May/2005, the authors developed a prospective, double-blinded and self-paired study analyzing abdominal CT images of 51 patients with clinical and laboratory diagnosis of acute pancreatitis assisted in the hospital emergency departments of our institutions. Inclusion criteria were: patients 18 or more years old, with clinical and laboratory diagnosis of acute pancreatitis, and symptoms onset < 72 hours before the CT examination. The diagnosis was based on the presence of clinical signs of acute pancreatitis and increase in lipase and/or amylase serum levels (more than three times the normal values). Indication for CT was defined by the medical staffs responsible by the patients, according to the clinical routine of the respective hospitals, with no interference from the authors of the present study. Exclusion criteria were the following: contraindication for intravenous iodinated contrast agents according to protocols adopted by the respective CT units, history of recent blunt abdominal trauma or abdominal surgery (< 30 days). The present study was analyzed and approved by the Committees for Ethics in Research of the respective institutions, and the patients were given information and instructions about the examinations, and signed the appropriate term of informed consent. Examination technique CT studies were performed in helical equipment (Philips, Secural model; Philips, Tomoscan AV model; Elscint, Helicat Flash model), with axial, contiguous 5.0 or 7.0 mm-slices, from the diaphragm to the pubic symphysis, before and after intravenous iodinated contrast agent injection, and after oral administration of 1000 ml 5% iodinated contrast solution. Contrast-enhanced images acquisition was started 70 seconds after the beginning of intravenous bolus injection of iodinated contrast agent (portal phase), injection dose = 2.0 ml/kg up to 150 ml, by means of a injection pump, at an injection rate of 3.0 ml/s. All of the studies were recorded on appropriated radiological films. Analysis of CT images The CT images were independently evaluated by two radiologists (observers 1 and 2) specialized in abdominal CT and with more than five years of experience in this field. The images were interpreted with no previous knowledge on the patients' clinical history or objective prognostic signs. Initially, all of the unenhanced CT images were separately analyzed, and after, images of unenhanced and contrast-enhanced phases were evaluated in conjunction. For evaluation of the intraobserver reproducibility, the images were reviewed by the same observers, utilizing the same methodology, after at least 30 days from the first analysis. At each phase of analysis, images were classified according to the morphological index and peripancreatic findings described by Balthazar et al. in 1985(3) (Chart 1), separately for the unenhanced and contrast-enhanced phases, and according to the degree of pancreatic necrosis described by the same authors in 1990(8) (Chart 1) only for the contrast-enhanced phase.
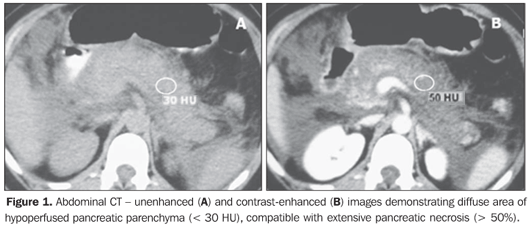
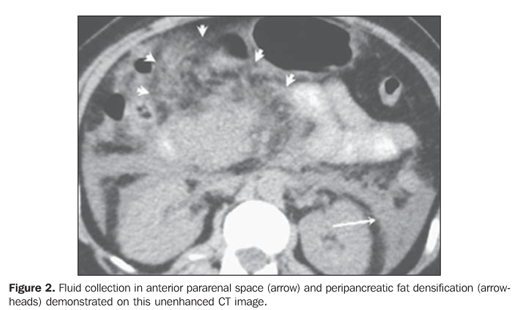
Pancreatic necrosis was characterized on contrast-enhanced CT images in the presence of a focal or diffuse, well-defined area of hypoperfused pancreatic gland tissue (enhancement < 30 UH) (Figure 1)(5,8). Peripancreatic inflammatory alterations were characterized by the presence of heterogeneity and densification(2,3,7,9). Peripancreatic collections were interpreted as well- or illdefined areas of fluid attenuation (< 20 UH)(2,3,7,9). Subjective criteria were adopted for differentiating between collected fluid and inflammatory fluid collections, considering that, due to the examination precociousness (up to 72 hours from symptoms onset), the fluid collections are still poorly encapsulated. Also, the number of fluid collections, presence of focal or diffuse pancreatic enlargement, parenchymal heterogeneity, and presence or absence of necrosis as well as its extent were established(2,3,7,9) (Figures 1 and 2).
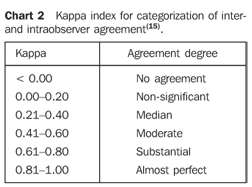
Statistical analysis The kappa index (k)(9,15) was utilized to measure interobserver and intraobserver agreement, according to Chart 2, comparing, respectively, data from each pair of observers, and data from each observer for the same parameters obtained at different moments. For this purpose, data from patients provided by both observers were divided into three groups according to the results from their analysis: mild acute pancreatitis (morphological index A or B and/or CT severity index 0 to 3), moderate acute pancreatitis (Morphological index C and/or CT severity index 4 to 6), or severe acute pancreatitis (morphological index D and/or CT severity index 7 to 10). As regards the presence of necrosis, the patients were divided into two groups: one without necrosis, and another with pancreatic necrosis. The same groups classification above described for CT severity index was utilized to evaluate the degree of pancreatic necrosis (Chart 1).
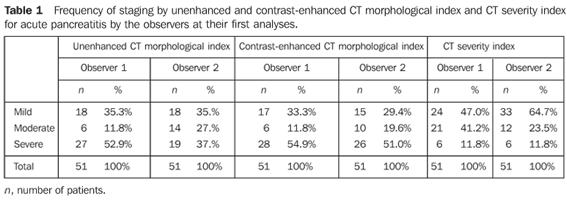
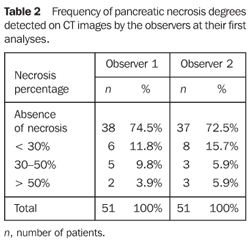
RESULTS The sample of 51 patients included 31 men and 20 women in the age range between 20 and 84 years (mean 49.5 ± 16.8 years). The disease etiology could be defined only for 31 patients: 17 of them had biliary pancreatitis, six had alcohol-induced pancreatitis, five had pancreatitis caused by drugs, two had pancreatitis resulting from endoscopic retrograde cholangiopancreatography (ERCP), and one caused by hyperlipidemia. The distribution of the tomographic classification by the observers in their first analyses is shown on Tables 1 and 2.
The comparison of results from the observers analyses (interobserver reproducibility) demonstrated substantial interobserver agreement for non-enhanced CT morphological indices (k = 0.666), contrast-enhanced CT morphological indices (k = 0.705), detection of necrosis (k = 0.648), and for CT severity indices (k = 0.631); moderate interobserver agreement was found for degree of necrosis (k = 0.547). The comparison of results from observers analyses (respectively 1 and 2) at different moments (intraobserver reproducibility) demonstrated substantial intraobserver agreement for unenhanced CT morphological indices (k = 0.796 and 0.732); contrast-enhanced CT morphological indices (k = 0.725 and 0.802); respectively, substantial and almost perfect intraobserver agreement for identification of necrosis (k = 0.674 and 0.849); respectively, moderate and substantial intraobserver agreement for degree of necrosis (k = 0.606 and 0.770); and substantial intraobserver agreement for CT severity indices (k = 0.801 and 0.687).
DISCUSSION The high reproducibility of a prognostic method is essential and desirable, considering that it allows the comparison between subsequent follow-up examinations, clinical tests, and between different clinical centers. Despite their widespread utilization, the parameters for calculating acute pancreatitis severity by means of CT are subjective, and therefore susceptible to variations between different observers or even by a same observer at different moments. Some authors, such as Lecesne et al.(9), in 1999, and Mortele et al.(10,11), in 2004, evaluated the CT reproducibility by measuring the interobserver agreement. These studies demonstrated moderate to substantial for calculation of CT severity index. However they failed to evaluate the intraobserver reproducibility and are likely to be criticized for their incompleteness. In all of the other studies evaluating the role of CT in the prognostic evaluation of acute pancreatitis severity, the tomographic scoring was performed on an individual basis or by consensus. Main parameters susceptible to variation in the interpretation of the CT severity index are the definition of the number of fluid collections and the pancreatic necrosis extent. In an attempt to minimize this variability, Mortele et al.(10) have devised a modified CT severity index, based on a different classification of inflammatory alterations and necrosis extent, disregarding the number of fluid collections and considering the necrosis extent as smaller or larger than 30%. Better results have been achieved in the prediction of acute pancreatitis severity by the modified CT severity index, but, contrarily to the expected results, the level of agreement was similar to the traditional CT severity index. The data of the present study demonstrated a substantial agreement for definition of unenhanced and contrast-enhanced CT morphological index, presence or absence of necrosis, and CT severity index. The only parameter presenting a little less consistent agreement (moderate) was the definition of pancreatic necrosis extent, although it has not affected the results of agreement in CT severity index. In the present study, the interobserver agreement comparable to or even higher than the interobserver agreement in the other three studies (9-11) previously developed for defining the CT severity index (the only parameter analyzed by these studies), since they have achieved a moderate to substantial agreement. As regards the intraobserver reproducibility, substantial to almost-perfect agreement was observed for all the parameters, except for necrosis extent by one of the observers (observer 1) who obtained a moderate agreement for this parameter. In the literature review, the authors have not found any study evaluating the reproducibility of definition of both unenhanced and contrast-enhanced CT morphological indices, and definition of presence of pancreatic necrosis extent. Also, no study was found on the intraobserver reproducibility for all of these parameters and CT severity index. The results from the present study indicate that unenhanced or contrast-enhanced CT constitute quite reproducible method, provided they are in the hands of experienced radiologists. Some limitations of the present study should be highlighted: the number of patients could be higher, but the size of sample evaluated is similar to the average sample in other studies. The correlation between acute pancreatitis staging, laboratory tests results and clinical progression was not established, considering that this correlation had been already demonstrated by other authors(2,3,8). Finally, the present study measured the method reproducibility utilizing only experienced observers.
CONCLUSIONS Computed tomography is a highly reproducible method in the determination of morphological index and disease severity index for staging of acute pancreatitis. The absence of contrast-enhancement does not affect the computed tomography morphological and severity indices reproducibility that remains high. A lower reproducibility is observed for definition of pancreatic necrosis which, however, does not affect the reproducibility of the CT severity index calculation.
REFERENCES 1. McKay CJ, Imrie CW. Staging of acute pancreatitis. Is it important? Surg Clin North Am 1999; 79:733–743. [ ] 2. Balthazar EJ. Acute pancreatitis: assessment of severity with clinical and CT evaluation. Radiology 2002;223:603–613. [ ] 3. Balthazar EJ, Ranson JH, Naidich DP, Megibow AJ, Caccavale R, Cooper MM. Acute pancreatitis: prognostic value of CT. Radiology 1985;156: 767–772. [ ] 4. Triviño T, Lopes Filho GJ, Torrez FRA. Pancreatite aguda: o que mudou? GED Gastrenterol Endosc Digest 2002;21:69–76. [ ] 5. Elias Júnior J. Utilização da tomografia computadorizada sem contraste e da ressonância magnética no diagnóstico e na estratificação da gravidade da pancreatite aguda. (Tese de Doutorado). Ribeirão Preto, SP: Faculdade de Medicina de Ribeirão Preto – Universidade de São Paulo, 2002. [ ] 6. van den Biezenbos AR, Kruyt PM, Bosscha K, et al. Added value of CT criteria compared to the clinical SAP score in patients with acute pancreatitis. Abdom Imaging 1998;23:622–626. [ ] 7. De Sanctis JT, Lee MJ, Gazelle GS, et al. Prognostic indicators in acute pancreatitis: CT vs APACHE II. Clin Radiol 1997;52:842–848. [ ] 8. Balthazar EJ, Robinson DL, Megibow AJ, Ranson JH. Acute pancreatitis: value of CT in establishing prognosis. Radiology 1990;174:331–336. [ ] 9. Lecesne R, Taourel P, Bret PM, Atri M, Reinhold C. Acute pancreatitis: interobserver agreement and correlation of CT and MR cholangiopancreatography with outcome. Radiology 1999;211: 727–735. [ ] 10. Mortele KJ, Wiesner W, Intriere L, et al. A modified CT severity index for evaluating acute pancreatitis: improved correlation with patient outcome. AJR Am J Roentgenol 2004;183:1261–1265. [ ] 11. Mortele KJ, Mergo PJ, Taylor HM, et al. Peripancreatic vascular abnormalities complicating acute pancreatitis: contrast-enhanced helical CT findings. Eur J Radiol 2004;52:67–72. [ ] 12. Schroder T, Kivisaari L, Somer K, Standertskjold-Nordenstam CG, Kivilaakso E, Lempinen M. Significance of extrapancreatic findings in computed tomography (CT) of acute pancreatitis. Eur J Radiol 1985;5:273–275. [ ] 13. Casas JD, Diaz R, Valderas G, Mariscal A, Cuadras P. Prognostic value of CT in the early assessment of patients with acute pancreatitis. AJR Am J Roentgenol 2004;182:569–574. [ ] 14. Freire Filho EO, Jesus PEM, D'Ippolito G, Szejnfeld J. Tomografia computadorizada sem contraste intravenoso no abdome agudo: quando e por que usar. Radiol Bras 2006;39:51–62. [ ] 15. Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics 1977;33:159–174. [ ]
Received November 4, 2006. Accepted after revision April 9, 2007.
* Study developed at Department of Imaging Diagnosis, Universidade Federal de São Paulo/Escola Paulista de Medicina (Unifesp/EPM), and Hospital e Maternidade São Luiz, São Paulo, SP, Brazil. |
|
Av. Paulista, 37 - 7° andar - Conj. 71 - CEP 01311-902 - São Paulo - SP - Brazil - Phone: (11) 3372-4544 - Fax: (11) 3372-4554