Radiologia Brasileira - Publicação Científica Oficial do Colégio Brasileiro de Radiologia
AMB - Associação Médica Brasileira CNA - Comissão Nacional de Acreditação
 Vol. 40 nº 6 - Nov. / Dec. of 2007
Vol. 40 nº 6 - Nov. / Dec. of 2007
|
WHICH IS YOUR DIAGNOSIS?
|
|
Qual o seu diagnóstico? |
|
|
Autho(rs): Marcelo Souto Nacif, Eduardo Benchimol Saad, Luiz Eduardo Montenegro Camanho, Fernanda d'Araujo Costa Ferreira, Ieda Prata Costa, Amarino Carvalho de Oliveira Júnior |
|
|
IProfessor, Faculdade de Medicina de Teresópolis (Unifeso), Teresópolis, RJ, Sub-coordinator of Post-Graduation, Instituto de Pós-Graduação Médica Carlos Chagas (IPGMCC), Fellow PhD degree in Radiology (Cardiac MRI), Universidade Federal do Rio de Janeiro (UFRJ), MD, Radiologist, Department of Radiology and Imaging Diagnosis, Hospital Pró-Cardíaco, Rio de Janeiro, RJ, Brazil
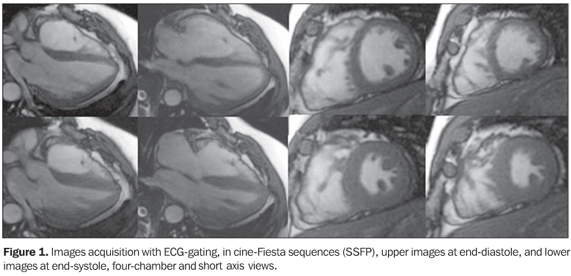
A male, 43-year old patient weighting 103 kg, with 1.70 m in height, presenting with frequent ventricular arrhythmias, who had experienced three episodes requiring electrical cardioversion, was referred to Hospital Pró-Cardíaco, Depart ment of Radiology and Imaging Diagnosis, to be submitted to cardiac magnetic resonance imaging (MRI). Images description Figure 1. Electrocardiographic (ECG) gating acquisitions in cine-Fiesta sequences (SSFP), upper images at end-diastole, and lower images at end-systole, four-chamber and short axis views. Note the normal-sized atriums. The left ventricle (LV) presented with preserved global and segmented functions, with 65% ejection fraction. The right ventricle (RV) presented with global dilatation, slight dysfunction, and estimated 32% ejection fraction (Simpson). Thinned RV wall with slight parietal irregularities is observed near the outflow tract.
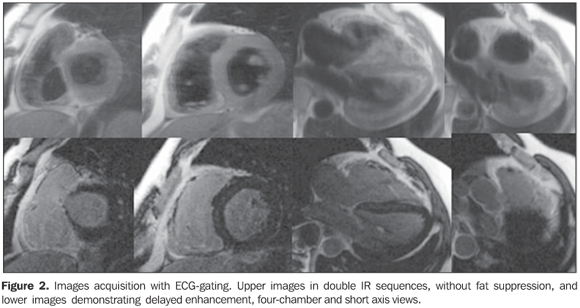
Figure 2. Images acquisition with electrocardiographic (ECG) gating. Upper images in double IR sequences, without fat suppression, and lower images demonstrating delayed enhancement, four-chamber and short axis views. Small foci of fibrofatty replacement are observed, characterized by the correlation between the two sequences where delayed myocardial enhancement can be identified on the RV wall, near the outflow tract.
Diagnosis: Arrhythmogenic right ventricular dysplasia (ARVD).
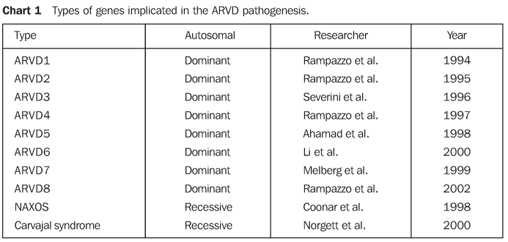
COMMENTS ARVD, also known as right ventricular cardiomyopathy (RVC) because of structural alteration of the cardiac muscle, has first been identified in a group of patients submitted to surgical intervention for ventricular tachycardia who had not responded to the treatment with antiarrhythmic drugs and with no history of heart disease(1,2). ARVD is characterized by a progressive fibrolipomatous replacement of myocardial cells intermingled with normal myocytes(3). A great number of cases of ARVD present a familial distribution, and, most frequently this disease is observed in some specific geographical regions of the world. Some genes implicated in the onset of this cardiomyopathy have already been described (Chart 1).
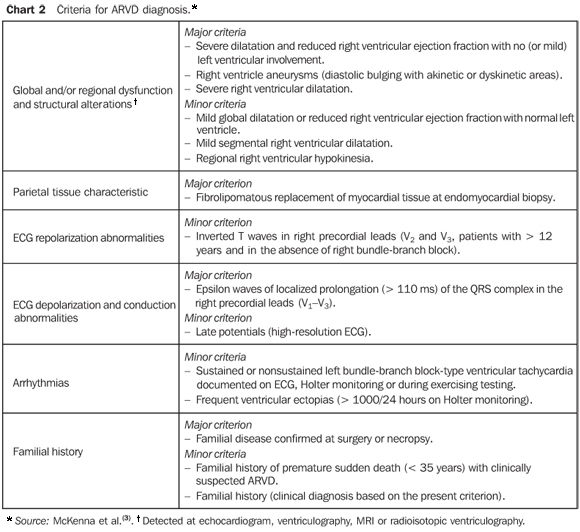
ARVD is a rare disease with an estimated incidence of 1:5000, predominantly affecting young men with no history of cardiovascular disease. It is characterized by frequently severe ventricular arrhythmias associated with sudden cardiac death, particularly during physical activity. Palpitation, dizziness, and even syncope are the main symptoms of the disease as a result of ventricular tachycardia and, less frequently, related to ventricular extrasystole. Another relevant and paradoxical aspect is the frequent presence of a practically normal clinical examination during sinusal rhythm(2,4-6). ARVD is considered as a progressive condition that may potentially lead to heart failure and death over a variable period of time. The diagnosis is based on internationally accepted clinical and laboratory criteria. The presence of two major criteria, one major and two minor criteria, or four minor criteria from different categories is considered as a diagnostic proof of the disease (Chart 2)(3,5,7-9).
High-resolution ECG As it can be seen on Figure 3, some relevant findings were observed on the high-resolution ECG of this patient, with inverted T waves in right precordial leads, late low amplitude potentials and high frequency at the end of the electrical ventricular activity, and left bundle-branch block-type ventricular tachycardia. Cardiac magnetic resonance imaging (CMR) Presently, CMR has become the standard for evaluation of anatomic and both RV segmental and global functions, playing a significant role in the diagnosis and follow-up of ARVD(5,10). This method allows a morphological analysis of both ventricles and their contractile activities by means of ECG gating acquisitions in cine-Fiesta sequences, demonstrating cavitary diameters and ventricular indices(10). In the present case, besides thinned RV wall with slight parietal irregularities observed near the outflow tract, the following data were obtained: RV short axis - 5.2 cm (normal: 2.2 cm to 4.4 cm); 32% RV ejection fraction (normal: 40% to 60%); end-diastolic RV volume - 98 ml/m2 (normal: 62 ml/m2 to 88 ml/m2); and end-systolic RV volume - 65 ml/m2 (normal: 10 ml/m2 to 30 ml/m2); besides the normal LV ejection fraction, characterizing the presence of two minor diagnostic criteria. The demonstration of fatty tissue infiltration into the ventricular wall in dark-blood sequences with and without fat-suppression (double and triple IR) may be performed and interpreted with a certain degree of confidence, besides allowing the identification of the fibrofatty tissue in the analysis of the delayed-enhancement images, demonstrating the presence of gadolinium in the fibrotic myocardium. In the present case, the presence of foci of fibrofatty replacement on the RV wall has been observed near the outflow tract, with delayed myocardial enhancement, characterizing the presence of fibrosis. Virtually, this data represents a major diagnostic criterion, considering that, according to the literature, this finding should only be characterized with directed biopsy. However, some authors like Auffermann et al.(4), suggest the replacement of angiography and, possibly, biopsy by CMR in the diagnosis of ARVD. Because of the CMR capacity to detect fibrofatty tissue, this method has been considered as capable of diagnosing ARVD based on the recognition of the histopathological marker of this disease(8). However, theoretical and practical issues should be taken into consideration: 1 - The presence of fatty tissue in the RV is found in different situations, including in healthy individuals. This has been already proved by Kaminaga et al.(7), who, quantitatively analyzing fatty tissue in the ventricular myocardium of 345 patients with several cardiopathies, have demonstrated that the prevalence of fatty tissue is 6% in ischemic cardiopathy, 7% in Kawasaki disease, 11% in hypertrophic cardiomyopathy, 18% in idiopathic dilated cardiomyopathy, and 33% in cases of ARVD. 2 - In spite of the good correlation between the presence of foci of fibrosis in the RV and histopathological findings, and the value of this finding as a predictor of episodes of tachycardia during electrophysiological studies, the technical limitations associated with spatial resolution still do not allow the detection of small fatty or fibrofatty deposits. 3 - Patients who have recently been submitted to procedures involving ablation or cauterization of the RV wall, may present non-specific foci of delayed enhancement as a result of these previous procedures. Therefore, MRI can be considered as an useful tool in the diagnosis of ARVD in more advanced stages of the disease with a more diffuse and extensive RV involvement. It is important to note that the presence of parietal fat may represent an anatomical variation in normal myocardiums(6). Presently, the detection of fat and fibrosis by CMR is less relevant than in the past, with functional and morphological alterations like the presence of aneurysms, "serrations" or trabecular disorders being more significant as far as the ARVD diagnosis is concerned(9,10). Final considerations Experience is an essential factor for a correct interpretation of RV findings, considering that typical variations of the RV are more significant than LV variations. Therefore, isolated CMR findings should be carefully interpreted and correlated with the other diagnostic criteria in order to avoid erroneous affirmative interpretation. In the present case, besides the identification of fibrofatty tissue and slight RV dilatation with reduced ejection fraction, three additional minor criteria (by high-resolution ECG) were found, complementing the diagnostic criteria.
REFERENCES 1. Corrado D, Leoni L, Link MS, et al. Implantable cardioverter-defibrillator therapy for prevention of sudden death in patients with arrhythmogenic right ventricular cardiomyopathy/dysplasia. Circulation 2003;108:3084–3091. [ ] 2. Elias J, Tonet J, Frank R, Fontaine G. Displasia arritmogênica do ventrículo direito. Arq Bras Cardiol 1998;70:449–456. [ ] 3. McKenna WJ, Thiene G, Nava A, et al. Diagnosis of arrhythmogenic right ventricular dysplasia/cardiomyopathy. Task Force of the Working Group Myocardial and Pericardial Disease of the European Society of Cardiology and of the Scientific Council on Cardiomyopathies of the International Society and Federation of Cardiology. Br Heart J 1994;71:215–218. [ ] 4. Auffermann W, Wichter T, Breithardt G, Joachimsen K, Peters PE. Arrhythmogenic right ventricular disease: MR imaging vs. angiography. AJR Am J Roentgenol 1993;161:549–555. [ ] 5. Midiri M, Finazzo M, Brancato M, et al. Arrhythmogenic right ventricular dysplasia: MR features. Eur Radiol 1997;7:307–312. [ ] 6. Fontaliran F, Fontaine G, Fillette F, Aouete P, Chomette G, Grosgogeat Y. Frontières nosologiques de la dysplasie arythmogène. Variations quantitatives du tissu adipeux ventriculaire droit normal. Arch Mal Coeur 1991;84:33–38. [ ] 7. Kaminaga T, Naitou H, Hamada S, Takamiya M. Detection of myocardial fatty components with ultrafast CT. Nippon Igaku Hoshasen Gakkai Zasshi 1993;53:28–34. [ ] 8. Castillo E, Tandri H, Rodriguez ER, et al. Arrhythmogenic right ventricular dysplasia: ex vivo and in vivo fat detection with black-blood MR imaging. Radiology 2004;232:38–48. [ ] 9. Tandri H, Friedrich MG, Calkins H, Bluemke DA. MRI of arrhythmogenic right ventricular cardiomyopathy/dysplasia. J Cardiovasc Magn Reson 2004; 6:557–663. [ ] 10. Rochitte CE, Pinto IMF, Fernandes JL, et al. I Diretriz de Ressonância e Tomografia Cardiovascular da Sociedade Brasileira de Cardiologia. Arq Bras Cardiol 2006;87:e48–e59. [ ]
Study developed at Hospital Pró-Cardíaco, Rio de Janeiro, RJ, Brazil. |
|
Av. Paulista, 37 - 7° andar - Conj. 71 - CEP 01311-902 - São Paulo - SP - Brazil - Phone: (11) 3372-4544 - Fax: (11) 3372-4554