Radiologia Brasileira - Publicação Científica Oficial do Colégio Brasileiro de Radiologia
AMB - Associação Médica Brasileira CNA - Comissão Nacional de Acreditação
 Vol. 40 nº 2 - Mar. / Apr. of 2007
Vol. 40 nº 2 - Mar. / Apr. of 2007
|
ORIGINAL ARTICLE
|
|
Microscopical analysis of fractionated cobalt-60 radiotherapy effects on mandibles of rats |
|
|
Autho(rs): Audrei Pelisser, Fabiana Vieira Vier-Pelisser, Vânia Regina Camargo Fontanella, Maria Antonia Zancanaro de Figueiredo |
|
|
Keywords: Radiotherapy, External beam radiation therapy, Late effects, Radiation damage, Osteoradionecrosis, Rats |
|
|
Abstract:
IMaster Degree in Oral & Maxillofacial Traumatology and Surgery by Universidade Luterana do Brasil, Canoas, RS, Brazil
INTRODUCTION Radiotherapy, a modality utilized for treatment of head andneck cancer(1), employs ionizing radiations tocontrol neoplastic cells, as a curative or adjuvant treatment ofthe malignant lesion(2). The radiation doseranges between 40 Gy and 70 Gy, and usually is fractionated indaily doses of about 2 Gy, allowing the distribution of therecommended total dose over a period of four to sevenweeks(3). Besides the radiation therapeutical effects, specificcomplications may arise following irradiation of this region,such as cavities, limitation of the mouth opening degree,decrease in the quality of the masticatory function anddysgeusia(4,5). Alterations resulting from the ionizing radiation action onthe bone tissue, which cause the development ofosteoradionecrosis, are considered as the most severecomplication from head and neckradiotherapy(6), and must be studied. Marx(7) has defined osteoradionecrosis as ametabolic and hemostatic deficiency resulting fromradiation-induced tissue injure. Osteoradionecrosis is characterized by the following sequence:irradiation, hypoxia, hypovascularization and hypocellularity oftissues. Irradiation affects the endothelium, and causeshyalinization and blood vessels thrombosis. The periosteumbecomes fibrotic, osteocytes and osteoclasts become necrotic byfibrosis of medullary spaces(7–10). Thecapacity of replacing collagen and normal bone cells inirradiated tissues is extremely affected or does notexist(7). Because of the reducedvascularization and osteocytes destruction, osteoradionecrosisoccurs in approximately 20% of patients who underwent localirradiation(3). Baker(11) has reported the relationshipbetween the tissues response to radiation and the capacity ofrepairing or not radio-induced lesions. Fast-response tissues arethose which present clinical manifestation of lesions in a shorttime interval after irradiation like skin, mucosas, hematopoietictissue, lymphoid tissue, and certain tumors. The slow-responsetissues are those which present more delayed responses. Examplesof this kind of tissue are: bone, conjunctive, muscular andnervous tissues with low proliferative activity. Meyer et al.(12) have compared theorthovoltage (200 kV) and supervoltage (cobalt-60) effects on 70three-month old rats, with 10 Gy, 15 Gy and 20 Gy doses. The bonetissue was studied in the interdental septum region between thefirst and the second lower molars. In the animals receivingorthovoltage the bone presented an irregular structure, withdegenerative sites. Pyknosis was observed in osteocytes.Osteocytes presence was not evidenced on the distal septalmargin, and a considerable decrease in the number of osteoblastswas observed. Medullary spaces were enlarged, showing decrease inthe cellular components density. In the animals irradiated withcobalt-60, the bone was essentially normal. Occasionally, someanimals showed a decrease in the number of osteoblasts andfibroblasts adjacent to the periodontal membrane. Rohrer et al.(13) have evaluated the effectsof high radiation-doses on the mandibles of eight monkeys. Theanimals were irradiated with a total 45 Gy dose of cobalt-60,fractionated into ten sessions over a 12-day period, equivalentto 70 Gy divided into 35 sessions over seven weeks. The animalswere sacrificed within one week to six months after the treatmentcompletion. Histological cuts were performed in the molars region(localized in the irradiation field), and in the incisives region(non-irradiated). The molars region was subdivided into cortical,Haversian and medullary bone. At each histological cut, thepercentage of empty osteocytic lacunae was recorded. Osteocyteswere absent in the cortical bone lacunae and in the Harvesianbone, presenting mean values of respectively 35% and 32%.Starting on the third month, the presence of empty lacunae wasmore remarkable. However, the values for the medullary bone weresimilar to those in the control group. The irradiated medullaryportion of the mandibles showed a marked proliferation. Theperiosteum inside the irradiation field showed loss ofcellularity, of vascularization, and inhibition of osteoidformation. The bone-marrow of the irradiated animals showedmarked alterations, including fibrosis, proliferation of new bonetissue and endoarteritis obliterans. For the authors, thedifference in percentages of empty lacunae in distinct regions ofthe bone tissue may be a result of the local regenerativecapacity and variation of blood supply. For Baker(11), the initial changes in theirradiated bone tissue result in a decrease in the osteocytespopulation. Osteoblasts tend to be more radiosensitive thanosteoclasts, so after a radiotherapy course it should there be ahigher disproportionality of the lytic activity. With theexcessive reduction of osteocytes, bone regions becomedevitalized and degenerative alterations start to develop. Thesechanges are potentialized because radiation also causes injuriesto small blood vessels in the bone, as well as to the oralmucosa. Lambert et al.(14) have reported thattherapeutic radiation-doses induce death of endothelium,thrombosis and blood vessels hyalinization. The progressivevessels obliteration results in decreased microcirculation. Theperiosteum of an irradiated bone becomes fibrous, withdestruction of osteoblasts and osteocytes, and spaces appear inthe bone marrow and start being filled with fibrous tissue. For Sykes(15), the bone cells and tissuesvascularization become irreversibly damaged when irradiated, witha consequent devitalization of the bone tissue. Based in this brief literature review, one may observe thatradiation on the region of head and neck causes damages to thevascularization and affects the cellularity of the bone tissuewhich becomes potentially susceptible to osteoradionecrosis.Although innumerable studies in the literature have mentioned theoccurrence of hypoxia, hypocellularity and hypovascularity in theirradiated bone tissue(7–10), there are fewstudies(13) evaluating quantitatively thealteration in the number of osteocytes in this tissue. Therefore, the present study is aimed at evaluating the meanpercentage of osteoblasts in the mandibular tissue of ratssubmitted to fractionated cobalt-60 radiotherapy, immediatelyafter the completion of the radiotherapy scheme and in thesubsequent 30 days, comparing the results with non-irradiatedanimals.
MATERIALS AND METHODS The present study had its protocol approved by the ScientificCommitter for Ethics of Faculdade de Odontologia da UniversidadeLuterana do Brasil, Canoas, RS, Brazil. The study sample included 45 male, 80-day old albine rats(Rattus norvegicus), of the Wistar strain, originatingfrom the biotery of Universidade Federal do Rio Grande do Sul,Porto Alegre, RS, Brazil, and weighting 220–290 g at thebeginning of the experiment. During the experimental period, theanimals received Labina-type solid diet and water adlibitum and were randomly distributed into three groups:group 1 (experimental group 1) – 15 animals submitted to 60 Gyradiation-dose, and sacrificed at the end of the radiotherapy;group 2 (experimental group 2) – 15 animals submitted to 60 Gyradiation-dose, and sacrificed 30 days after the radiotherapycompletion; group 3 (control group) – 15 non-irradiated animals,subdivided into two groups with, respectively, seven rats for theexperimental group 1 (group 3a), and eight for the experimentalgroup 2 (group 3b). The animals in groups 1 and 2 were irradiated in the Service of Radiotherapy at Hospital São Lucas – Pontifícia Universidade Católica do Rio Grande do Sul, RS, Brazil, employing a cobalt-60 teletherapy unit with rotational gantry (Philips, XK 5101 model), with 1.25 MeV energy. The total dose employed was 60 Gy, divided into 30 2Gy-fractions, delivered during 15-minute daily sessions, from Mondays to Fridays, over a 6-week period(16,17). The distance between the radiation beam emitter and the animals skin surface was 60 cm (Figure 1A). The head and neck region was placed in the irradiation field measuring 20 cm × 20 cm, with the animals immobilized during the session by means of plastic devices (Figure 1B).
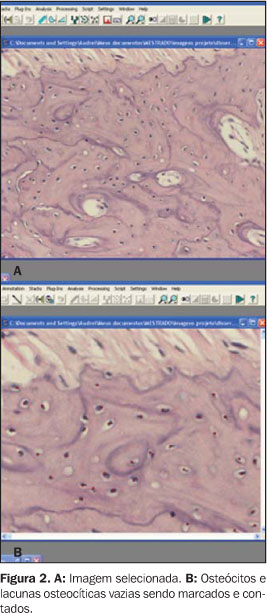
Animals of groups 1 and 3a were sacrificed 60 hours after theteletherapy completion. Animals of groups 2 and 3b were equallysacrificed 30 days after. After intraperitoneal anesthesia withsodic tiopental and S+ ketamine hydrochloride at 22 mg/kg, theanimals were perfused with 4% paraformaldehyde. Previously to theemployment of the fixative solution, the animals head circulatorysystem was washed with 50 ml 0.1 M, 7.3 pH phosphate bufferedsaline solution The left hemimandible of the animal was dissected, and afragment containing the three molar teeth and the middle third ofthe incisive was submitted to evaluation by light microscopy. Thesamples were decalcified in ethylenediamine acetic acid at 17%(pH 7.0). The specimens were processed by the routine technique,taking care to keep the plane formed by long axis of the threemolar teeth parallel to the plane of one of the block surfaces.The histological sheets were hematoxylin-eosin stained and threeserial 5 µm thick cuts were obtained. The site of evaluation in the histopathological cuts was themedullary bone, near the roots of the first and second lower leftmolars. Two areas of each histological sheet were selected,corresponding to the regions of first and second molars. Imagescapture was performed with an Olympus AX-70 optic microscopecoupled with a system of digital images capture, with 200xmagnification. The software Image-Pro Plus version 4.0 wasutilized for the images storage. Images were entered into the ImageTool software, for osteocytes and osteoplasts counting (Figure 2) by means of the tag and count resource. The image was divided into quadrants to be magnified in order to facilitate the counting. This count was repeated twice for each image. Numeric values were recorded in tables. Afterwards, an arithmetic mean of the two counts was calculated.
Aiming at evaluating the interexaminer concordance between thefirst and the second counts, the intraclass correlationcoefficient was utilized. The variance analysis, supplemented bythe Tukey's multiple comparison test at 5% significance level,was employed to evaluate the difference between the groupsconcerning the percentage of osteoplasts in tissues, with theSPSS software version 8.0.
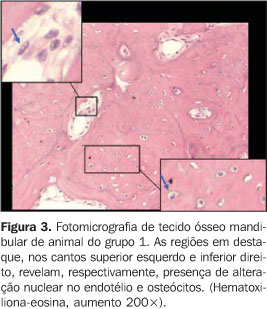
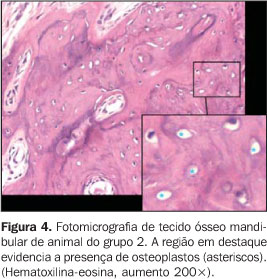
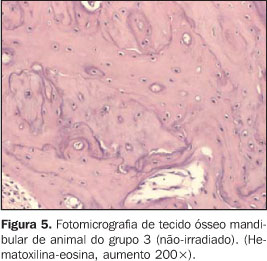
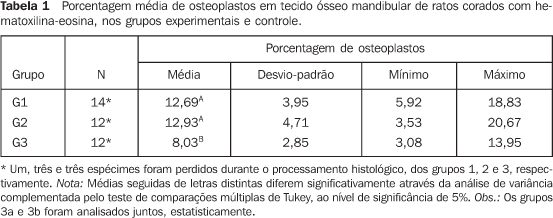
RESULTS There was no animal death during the radiotherapy scheme. Oneanimal in group 2 died during the 30-day interval, thewaiting-time between the radiotherapy completion and theeuthanasia of the animals in this group. During the treatment, the animals presented with a debilitatedappearance. Some of them demonstrated a visible decrease in thequantity of hair on the head, i.e., the radiation portal area, afact more clearly observed in the animals of group 2 which werekept alive up to 30 days after the radiotherapy completion. The intraclass correlation coefficient demonstrated a goodreproducibility between the first and the second measurement(ri = 0.956; p < 0.001). The animals in groups 1 and 2 (irradiated) did not differ from each other, presenting higher rates of osteoplasts (Figures 3 and 4) when compared with group 3 (control) (Figure 5) (Tables 1 and 2), according to the variance analysis supplemented by the Tukey's multiple comparison test at a 5% significance level.
The nuclei of some endothelium and osteocytes cells of the irradiated animals presented alterations similar to vacuoles or bubbles, or a spherical, slightly stained region (Figure 3).
DISCUSSION A concern that was present during the pilot project of thisexperiment was the necessity, or not, of anesthesia of theanimals during the radiotherapy sessions for them to keep theirheads towards the radiation area. However, while the anestheticprocedure aided in the restrain or displacement of the rats, ahigh number of applications could induce theirdeath(13,18). Consequently there would be adecrease in the size of the sample, considering that the protocoldefined 30 radiotherapy sessions. Fractionated radiotherapy hasbeen utilized in many studies with animals without anesthesiaduring treatment sessions(19–22). On the otherhand, studies performed with monkeys by Hutton etal.(23), Nickens et al.(24)and Rohrer et al.(13), followed a protocolestablishing that the anesthetic procedure was performed at eachradiation dose. Even the studies of Sweeney etal.(25), Zywietz et al.(26)and Sagowski et al.(16,17) utilizing rats asanimal models share this methodology. Although employing a single 15 Gy x-radiation dose in rats,English et al.(27) were the first authors todescribe the form of animals immobilization during theradiotherapy, without using anesthesia. The animals were placedinside a cylindrical tube whose anterior extremity had a holewith 1.0 cm–1.5 cm to allow the insertion of the animal nose.After that, a rubber lid with a tail slot was placed on theposterior extremity of the tube. The methodology employed bythese authors and by English(28) was a modelutilized for idealization of PET mineral water bottles used asrestrainers for the animals in the present study, eliminating thenecessity of anesthesia in the radiotherapy sessions. However,differently from the above mentioned authors, the apparatusdeveloped in this experiment allowed the exposure of the rat headthrough the anterior orifice of the bottle, facilitating theplacement of this region inside the irradiation field. A constant concern along the development of the present studywas the conformity of the experimental design with the clinicalreality. So, the radiotherapy dose employed in the animal modelwas the same employed in the protocol for treatment of oralneoplasms in humans(3). However, there was adoubt if the rat, as a function of its lower body volume comparedto the oncological patient, could support the usual radiotherapydose. But, analyzing the current literature, one may observe thatdoses of at least 60 Gy have been fractionatedly utilized inrats, employing different therapy modalities such asx-radiation(19, 21,22), cobalt-60(16,17,26) and thelinear accelerator(20). In the presentexperiment, the same protocol described by Sagowski etal.(16,17) was employed, i.e., 30 sessions ofcobalt-60 in 2 Gy-doses, totalling 60 Gy fractionated fromMondays to Fridays over a six-week period, which, in the finalanalysis, is the radiotherapy scheme utilized for treatment oforal cancer in humans. Radiotherapy, notwithstanding its beneficial effects on thetumor tissue localized in the head and neck region, causesinjuries to normal tissues localized in the radiation portal. So,salivary glands, oral mucosa, bone, teeth, masticatory musclesand temporomandibular joints are affected by ionizingradiation(5). The teletherapy effects on thedental pulp and submandibular gland could be observed in thestudies of Vier-Pelisser et al.(29) andVier-Pelisser(30). However, amongst radiotherapy late complications,osteoradionecrosis remains as a significant and serious clinicalproblem(31,32), because the irradiated bonepresents hypoxia, hypovascularization andhypocellularity(7–10). Despite the comments ofseveral authors on the destruction of osteocytes in irradiatedbone tissues(3,11,33), fewstudies(13) have performed a quantitativeanalysis of the reduction of these cells in the bone. The osteocyte occupies in the bone matrix a compartmentdenominated osteocytic lacuna orosteoplast(34). When this cell necrotizes, theosteocytic lacuna becomes empty. So, the objective of the presentstudy was to microscopically evaluate the mean percentage ofosteoplasts in mandibular bone tissue of rats submitted tofractionated cobalt-60 radiotherapy. Stone et al.(35) reported that the chronicradiation effects can be observed over months to years after thetherapeutic scheme completion. Taking this fact intoconsideration, the present study sought to evaluate the effectsof fractionated radiotherapy on mandibles of rats over twoexperimental periods: the first, immediately after theradiotherapy scheme completion, and the second, after the 30subsequent days, since the tissue metabolism of rats is fasterthan the humans'. The results of this investigation demonstrated that thepercentage of osteoplasts in booth experimental groups,independently from the observation period, was statisticallyhigher than the percentages in the control groups. This confirmsinnumerable authors assertions(3,7,9–13,22,33)reporting a hypocellularity condition in the tissue exposed toradiation. In this experiment, the decrease in the number of cells in theirradiated mandibular tissue was observed both immediately afterthe teletherapy completion and at the 30 subsequent days. Itshould be expected, however, that the percentage of osteoplastswould be higher in the experimental group 2, compared with group1, since osteoradionecrosis is a late effect of irradiation.Other studies employing observations over longer periods arenecessary to evaluate a possible worsening of the hypocellularityin this tissue. Considering the results of this research, one may ask thereason for the increase in the presence of osteoplasts in theirradiated tissue. Maybe, the damage observed on the terminalvascular bed, causing injury to the local microcirculation and tothe support stroma characterized by telangiectasia and occlusionof blood capillary vessels(11,14,33), causes adecrease in the vascularization(36), i.e, inthe blood supply to osteocytes which end up necrotizing. Theinjury in the local microcirculation also could be responsiblefor a decrease in the quantity of oxygen in the bone tissue.Additionally, the microcirculation seems to be more problematicin the mandible than in the maxilla. The latest typicallypresents more vascularized than the mandible, so theosteoradionecrosis incidence is higher in the mandibular than inthe maxillary tissue(31,37). In spite of not being the objective of the present study, onecould observe both in osteocytes and endotelial cells present inthe field of study, nuclear alterations characterized by theappearance of slightly stained regions similar to bubbles orvacuoles. These results corroborate the presence of damage fromthe irradiation to osteocytes and vascular endothelium. Thesesame nuclear alterations were observed in pulpal fibroblasts ofmolars in cobalt-60-irradiated rats(30). The decrease in the number of osteocytes in the irradiatedmandibular tissue of rats characterized by the increase in thepresence of osteoplasts would help to explain the occurrence ofosteoradionecrosis. Shafer et al.(33) reportedthat, in general, radiation, trauma and infection are factorsinvolved in the pathogenesis of this significant complicationfrom radiotherapy in the head & neck region. The recentliterature indicates a mechanism of fibroatrophy for developmentof osteoradionecrosis to the detriment of the traditionalmechanism of vascular insufficiency(32,38).Another hypothesis is that irradiation would lead toosteoradionecrosis by apoptosis induction in bonecells(39). It is thought that the decrease inthe number of osteocytes in the irradiated bone tissue is asignificant component in the etiology of osteoradionecrosis.
CONCLUSION Based on the methodology employed in the present study andrespective results, one may conclude that cobalt-60 teletherapyis capable of promoting a statistically significant increase(p = 0.005) in the mean percentage of osteoplasts inmandibular bone tissue of rats. This increase is observed bothimmediately after the completion of the radiotherapy scheme andin the subsequent 30 days.
REFERENCES 1. Silva JLF, Arruda FF. Radioterapia nos tumores de cabeça e pescoço – aspectos gerais. In: Guimarães JRQ. Manual de oncologia. São Paulo: BBS, 2004;475–488. [ ] 2. Lima AAS, Figueiredo MAZ, Loureiro MS, Duarte R. Radioterapia de neoplasias malignas na região da cabeça e pescoço – o que o cirurgião-dentista precisa saber. Rev Odonto Ciência 2001;16: 156–165. [ ] 3. Regezi JA, Sciubba JJ. Lesões inflamatórias dos maxilares. In: Regezi JA, Sciuba JJ. Patologia bucal. Correlações clinicopatológicas. 3ª ed. Rio de Janeiro: Guanabara Koogan, 2000;342–355. [ ] 4. Segreto HRC, Segreto RA. Revisão e atualização em radiobiologia. Aspectos celulares, moleculares e clínicos. A Folha Médica 2000;119:9–27. [ ] 5. Vissink A, Jansma J, Spijkervet FK, Burlage FR, Coppes RP. Oral sequelae of head and neck radiotherapy. Crit Rev Oral Biol Med 2003;14:199–212. [ ] 6. De Moor R. Influence directe et indirecte de la médication (chimiothérapie y comprise) et de l'irradiation sur la pulpe. Rev Belge Med Dent 2000; 55:321–333. [ ] 7. Marx RE. Osteoradionecrosis: a new concept of its pathophysiology. J Oral Maxillofac Surg 1983; 41:283–288. [ ] 8. Neville BW, Damm DD, Allen CM, Bouquot JE. Doenças da polpa e do periápice. In: Neville BW, Damm DD, Allen CM, Bouquot JE. Patologia oral & maxilofacial. Rio de Janeiro: Guanabara Koogan, 1998;93–118. [ ] 9. Store G, Olsen I. Scanning and transmission electron microscopy demonstrates bacteria in osteoradionecrosis. Int J Oral Maxillofac Surg 2005; 34:777–781. [ ] 10. Store G, Eribe ER, Olsen I. DNA-DNA hybridization demonstrates multiple bacteria in osteoradionecrosis. Int J Oral Maxillofac Surg 2005;34: 193–196. [ ] 11. Baker DG. The radiobiological basis for tissue reactions in the oral cavity following therapeutic x-irradiation. A review. Arch Otolaryngol 1982; 108:21–24. [ ] 12. Meyer I, Shklar G, Turner J. A comparison of the effects of 200 kV radiation and cobalt-60 radiation on the jaws and dental structure of the white rat. A preliminary report. Oral Surg Oral Med Oral Pathol 1963;15:1098–1108. [ ] 13. Rohrer MD, Kim Y, Fayos JV. The effect of cobalt-60 irradiation on monkey mandibles. Oral Surg Oral Med Oral Pathol 1979;48:424–440. [ ] 14. Lambert PM, Intriere N, Eichstaedt R. Clinical controversies in oral and maxillofacial surgery: Part one. Management of dental extractions in irradiated jaws: a protocol with hyperbaric oxygen therapy. J Oral Maxillofac Surg 1997;55:268–274. [ ] 15. Sykes LM. An interim extraoral prosthesis used for the rehabilitation of a patient treated for osteoradionecrosis of the mandible: a clinical report. J Prosthet Dent 2001;86:130–134. [ ] 16. Sagowski C, Tesche S, Zywietz F, Wenzel S, Metternich FU. The radioprotectors amifostine and sodium selenite do not modify the radiosensitivity of rat rhabdomyosarcomas. Onkologie 2004; 27:54–57. [ ] 17. Sagowski C, Wenzel S, Jenicke L, et al. Reducing late toxicity with amifostine in fractionated irradiation of the rat salivary glands. HNO 2002; 50:822–828. [ ] 18. Nathanson A, Bäckström A. Effects of 60Co-gamma-irradiation on teeth and jaw bone in the rabbit. Scand J Plast Reconstr Surg 1978;12:1–17. [ ] 19. Carl UM, Beck-Bornholdt HP, Baumann M, Lorenzen J, Vogler H. Radiotherapy of the rhabdomyosarcoma R1H of the rat: postoperative radiotherapy. Int J Radiat Oncol Biol Phys 1990;18: 883–886. [ ] 20. Ceelen W, El Malt M, Cardon A, Berrevoet F, De Neve W, Pattyn P. Influence of preoperative high-dose radiotherapy on postoperative outcome and colonic anastomotic healing: experimental study in the rat. Dis Colon Rectum 2001;44:717–721. [ ] 21. Kleineidam M, Pieconka A, Beck-Bornholdt HP. Radiotherapy of the rhabdomyosarcoma R1H of the rat: influence of the time interval between two daily fractions during hyperfractionated radiotherapy. Radiother Oncol 1994;30:128–132. [ ] 22. Würschmidt F, Becker S, Maurer T, et al. Radiotherapy of the rhabdomyosarcoma R1H of the rat: recovery from radiation injury in tumour and skin. Radiother Oncol 1992;23:105–110. [ ] 23. Hutton MF, Patterson SS, Mitchell DF, Chalian VA, Hornback NB. The effect of cobalt-60 radiation on the dental pulps of monkeys. Oral Surg Oral Med Oral Pathol 1974;38:279–286. [ ] 24. Nickens GE, Patterson SS, El-Kafrawy AH, Hornback NB. Effect of cobalt-60 radiation on the pulp of restored teeth. J Am Dent Assoc 1977;94: 701–704. [ ] 25. Sweeney WT, Elzay RP, Levitt SH. Histologic effect of fractionated doses of selectively applied 60Co irradiation on the teeth of albino rats. J Dent Res 1977;56:1403–1407. [ ] 26. Zywietz F, Hahn LS, Lierse W. Ultrastructural studies on tumor capillaries of a rat rhabdomyosarcoma during fractionated radiotherapy. Acta Anat (Basel) 1994;150:80–85. [ ] 27. English JA, Schlack CA, Ellinger F. Oral manifestations of ionizing radiation. II. Effect of 200 KV. x-ray on rat incisor teeth when administered locally to the head in the 1,500 R. dose range. J Dent Res 1954;33:377–378. [ ] 28. English JA. Localization of radiation effects in rats' teeth. Oral Surg Oral Med Oral Pathol 1956; 9:1132–1138. [ ] 29. Vier-Pelisser FV, Amenábar JM, Cherubini K, Figueiredo MAZ, Yurgel LS. Análise microscópica do efeito da radioterapia fracionada em glândula submandibular de rato. Radiol Bras 2005;38: 409–414. [ ] 30. Vier-Pelisser FV. Efeito da teleterapia fracionada em polpa dentária de ratos – análise em microscopia óptica e eletrônica de transmissão. (Tese de Doutorado). Porto Alegre: Pontifícia Universidade Católica do Rio Grande do Sul, 2005. [ ] 31. Ang E, Black C, Irish J, et al. Reconstructive options in the treatment of osteoradionecrosis of the craniomaxillofacial skeleton. Br J Plast Surg 2003;56:92–99. [ ] 32. Teng MS, Futran ND. Osteoradionecrosis of the mandible. Curr Opin Otolaryngol Head Neck Surg 2005;13:217–221. [ ] 33. Shafer WG, Hine MK, Levy BM. Lesões físicas e químicas da cavidade bucal. In: Shafer WG, Hine MK, Levy BM. Tratado de patologia bucal. 4ª ed. Rio de Janeiro: Guanabara Koogan, 1987; 486–548. [ ] 34. Whitson SW. Osso. In: Ten Cate AR. Histologia bucal: desenvolvimento, estrutura e função. 5ª ed. Rio de Janeiro: Guanabara Koogan, 2001;101–122. [ ] 35. Stone HB, Coleman CN, Anscher MS, McBride WH. Effects of radiation on normal tissue: consequences and mechanisms. Lancet Oncol 2003; 4:529–536. [ ] 36. Mathes SJ, Alexander J. Radiation injury. Surg Oncol Clin N Am 1996;5:809–824. [ ] 37. Thorn JJ, Hansen HS, Specht L, Bastholt L. Osteoradionecrosis of the jaws: clinical characteristics and relation to the field of irradiation. J Oral Maxillofac Surg 2000;58:1088–1093. [ ] 38. Delanian S, Lefaix JL. The radiation-induced fibroatrophic process: therapeutic perspective via the antioxidant pathway. Radiother Oncol 2004; 73:119–131. [ ] 39. Szymczyk KH, Shapiro IM, Adams CS. Ionizing radiation sensitizes bone cells to apoptosis. Bone 2004;34:148–156. [ ]
Received September 5, 2006.
* Study developed in the Program of Post-graduation in Odontology at Universidade Luterana do Brasil, Canoas, RS, and Program of Doctorate in Clinical Stomatology at Pontifícia Universidade Católica do Rio Grande do Sul (PUCRS), Porto Alegre, RS, Brazil. |
|
Av. Paulista, 37 - 7° andar - Conj. 71 - CEP 01311-902 - São Paulo - SP - Brazil - Phone: (11) 3372-4544 - Fax: (11) 3372-4554