Radiologia Brasileira - Publicação Científica Oficial do Colégio Brasileiro de Radiologia
AMB - Associação Médica Brasileira CNA - Comissão Nacional de Acreditação
 Vol. 42 nº 6 - Nov. / Dec. of 2009
Vol. 42 nº 6 - Nov. / Dec. of 2009
|
ORIGINAL ARTICLE
|
|
Computer-assisted analysis of breast tumors texture on sonographic images of patients submitted to breast-conserving surgery |
|
|
Autho(rs): Carolina Maria de Azevedo, André Victor Alvarenga, Wagner Coelho de Albuquerque Pereira, Antonio Fernando Catelli Infantosi |
|
|
Keywords: Computer-assisted image processing, Computer-assisted image interpretation, Computer-assisted decision making, Recurrence, Breast ultrasonography |
|
|
Abstract:
IPhD, Titular Professor, Hospital Universitário Gaffrée e Guinle (HUGG), Rio de Janeiro, RJ, Brazil
INTRODUCTION Breast cancer recurrence tends to occur within a five-year period in approximately 5% to 10% of patients submitted to breastconserving surgery in any breast quadrant, either with or without adjuvant therapy(1-3). Such lesions are not frequently found in a two-year postoperative period, usually occurring between four and six years after surgery and radiotherapy, generally because of treatment failure. Most of times, these lesions are found in the surgical site or adjacent to the area covered by the instituted therapy(2,4,5). In cases where the recurrence is observed after six years and located in a site different from the original surgical site, the presence of a new primary tumor or multicentric lesions is most probable(2,5). The term bilateral breast cancer implies that a woman with a breast tumor is diagnosed with malignancy in the contralateral breast. According to the literature, the risk of a woman developing a tumor in the contralateral breast is 0.7%/year. Necropsy studies have demonstrated that 68% of treated cancers develop recurrence and occult cancer in the contralateral breast(6,7). Tumors occurring at the same time are named synchronous or simultaneous tumors. The diagnosis and treatment for metachronous or asynchronous tumors are not the same as for primary tumors. The majority of breast cancers have a ductal origin and demonstrate a mixed pattern with areas of lobular pattern. Metastases to the contralateral breast may occur latter or remain stable for a long time. A second primary tumor may be present as the initial cancer is being treated, but is not detected because of its slow growth (synchronous tumor). The second primary tumor occurs after five years or more(2,5,8). The high incidence of primary carcinoma in the contralateral breast has been reported in necropsy studies. Risk factors involved in breast tumors recurrence include the following: young patients, positive surgical margins, extensive intraductal component, lymph node invasion, vascular invasion, presence of suspicious microcalcification in the surgical site, nodule observed at ultrasonography, nodule size. Nodule size and lymph node invasion play a relevant role in the determination of the disease prognosis(5,9,10). Currently, chemotherapy and postoperative adjuvant radiotherapy have contributed to reduce the number of cases with tumor recurrence(6,9,10). In spite of the difficulty posed by the surgical scar to detect this type of lesion, 67% of lesions are identified at physical examination, and 35%-50% are detected at mammography. At ultrasonography, recurrence in the form of a nodule or mass is better visualized and tends to present acoustic shadowing or shaded borders because of scar/fibrosis or presence of microcalcifications(3,11,12). Computer-assisted analysis of breast tumors texture and morphological characteristics on sonographic images has been widely studied(13-20). However, most of such studies analyze the malignancy or benignity of sonographic findings without discussing the clinical origins of the lesions. This type of analysis is important considering that the development of computer-aided diagnosis (CAD) systems and the subsequent utilization of such systems in the clinical routine may allow, for example, the definition of diagnosis and prognosis of breast tumors recurrence(6). The present study is aimed at analyzing the performance of computer-aided methods for quantifying breast lesions texture on sonographic images developed by Alvarenga et al.(20), as applied to two groups of clinically different patients as follows: patients submitted to breast-conserving surgery either with or without tumor recurrence.
MATERIALS AND METHODS Patients' and images bank characteristics The present study was made with the collaboration of Instituto Nacional de Câncer (INCA/MS/RJ) and Programa de Engenharia Biomédica (COPPE) (Program of Biomedical Engineering) of Universidade Federal do Rio de Janeiro (UFRJ). The study was developed in the period from 2001 to 2004, at the Hospital do Câncer I (HC I-INCA), with the evaluation of 36 patients submitted to breast-conserving surgery and undergoing follow-up. The primary tumors of such patients were invasive carcinomas stages II and III. After the surgery the patients were submitted to radiotherapy and chemotherapy as required according the surgery results. For the purposes of the present study, and before undergoing mammography and ultrasonography, the patients answered a questionnaire concerning surgical and adjuvant therapy, previous and current clinical history. Additionally, all the patients were submitted to ultrasound-guided fine-needle aspiration and excision biopsy. Twelve of the 36 patients presented local recurrence with primary invasive tumor stages II and III. Among them, seven presented lymphatic and vascular invasion. One of the patients with lymphatic and vascular invasion presented metastasis to the ipsilateral axilla. Among the patients with tumor recurrence, 11 were menopausal women. On average, the time interval between the primary tumor diagnosis and recurrence was 4.5 years, the earliest recurrence occurring within one year and the latest within eight years. The patients' age range was between 33 and 80 years (mean, 53 years). No race variation was observed among the patients. As regards the type of surgery, three patients were submitted to lumpectomy and nine to quadrantectomy. Six cases of local recurrence occurred in the right breast, and six in the left breast. As regards the postoperative adjuvant therapy, two patients underwent only radiotherapy, two patients were submitted to breast-conserving surgery with no supplementary therapy, only three were submitted to chemotherapy and five were submitted to chemotherapy and radiotherapy. All the patients presented alterations at clinical examination, mammography and ultrasonography. Among the 24 patients who did not present recurrence in the surgical site, 3 presented a malignant lesion in the contralateral breast, 7 presented benign nodules (three cysts and four fibroadenomas), and 5 presented atypical non-nodular hyperplasia and nine, fibrocystic alterations. The three patients with malignant tumors were menopausal women and were submitted to radiotherapy and chemotherapy. The first patient had a primary in situ tumor with areas of comedocarcinoma in the left breast. In the contralateral breast, the tumor presented the same characteristics and histological type of the primary tumor, probably corresponding to a metastatic lesion developed after three years. The second patient presented a primary lesion in the right breast and a second asynchronous tumor in the left breast. The primary tumor was an invasive carcinoma and did not present microcalcifications, while the second did. The time interval was four years. The third patient presented primary lesion in the right breast. The primary tumor was an invasive carcinoma with microcalcifications. The tumor in her contralateral breast did not present any microcalcification. The time interval between the primary tumor and the second (probably a second primary tumor) was six years. Concerning the type of surgery, 11 were submitted to lumpectomy and 13 to quadrantectomy. Nine patients had lesions in the left breast, and 15 in the right breast. Three patients presented lesions in the contralateral breast, two of them in the right, and one in the left breast. The mammographic studies were performed in a GE 600T model (General Electric Medical Systems; Milwaukee, USA) equipment and ultrasonography studies in a Siemens Sonoline Sienna unit (Siemens Medical Systems; Erlangen, Germany) with a 7.5 MHz transducer, having 0.45 mm and 0.49 mm as axial and lateral resolution, respectively. The equipment parameters were adjusted by an experienced radiologist based on his diagnostic routine, considering that the adopted lesion texture parameters are not influenced by the equipment settings. Among the sonographic views routinely utilized for diagnostic evaluation, only the most representative one was selected for the diagnosis of the lesion that was classified by an experienced radiologist according to its sonographic features, with the image being recorded as a TIF file directly from the ultrasonography unit for later processing. Texture parameters Two mathematical concepts described in the literature - grey level co-occurrence matrix (GLCM) and complexity curve (CC)(21) - were utilized to quantify the characteristics of breast lesions texture on sonographic images of patients submitted to breast-conserving surgery. GLCM is a two-dimensional histogram (dimensions matrix G × G grey levels) of an image f(x, y) describing the occurrence of pairs of pixels with values i and j, separated by a certain distance d, towards a determined direction θ, with the image pixels being submitted to pixels pair analysis. Further details on this histogram implementation may be found at Al-Janobi(21). Each lesion was attributed with three matrices corresponding to three specific θ angles (0º, 45º and 90º) and a three-pixel d distance. Then, the matrices average was divided by the number of pixels present in the image. For the resulting matrix, the following parameters were calculated: entropy (coo), second angular moment (asm), standard deviation (std), contrast (con) and correlation (cor)(20). The calculation of the CC was based on the number of transitions (from 1 to 0 or from 0 to 1) occurring in a binary image (only to grey levels: 0 and 1), based on the original image for each grey level present in this image. The graphic demonstrating the number of transitions for each grey level is called CC. Further details on CC calculation may be found at Baheerathan et al.(22). For the CC calculation, five parameters were considered(20), namely: maximum transition values (mv), mean transition values (av), mean grey level values promediated by the number of transitions (sm), grey levels standard deviation promediated by the number of transitions (ssd) and transitions number entropy (ent). Both GLCM and CC present advantages in the quantification of different textures as compared with less complex techniques such as, for example, grey levels histogram, since the techniques described in the present study are sensitive to the spatial distribution of the grey levels. The implementation of the software Matlab® (Mathworks Inc.; Natick, USA) was required for the determination of GLCM and CC, as well as their respective parameters, allowing the selection of a region of interest on the image of the lesion and then calculating the lesion texture parameters. Further details may be found at Alvarenga et al.(20). Linear discriminant analysis (LDA) Based on the calculated texture parameters, the lesions were classified according to a statistical method known as linear discriminant analysis. Objectively, this method is aimed at defining the surface (straight line or plain, depending on the number of variables employed, two or three, respectively) that provides the best statistical separation between sets of values of the different parameters studied. The LDA was applied to the parameters (normalized between -1 and 1) based on the GLCM (5) and CC (5), for differentiating breast lesions in patients submitted to breast conserving surgery either with or without tumor recurrence. The linear discriminant analysis is utilized for classification and data dimensionality reduction, and is based on the maximization of the discrepancy ratio between classes and within each class, assuring maximum data separation. Further details on this technique may be found, for example, at Johnson & Wichern(23). Considering the limited number of images available, the authors have opted for not combining parameters, utilizing the technique leave-one-case-out(24) (this method provides statistical validity to the analysis). The parameter performance was evaluated utilizing the area Az (± standard error) under the ROC (receiver operating characteristic) curve that is the classic method for measuring the global performance, working simultaneously with sensitivity and specificity(25). In an ideal case, i.e., 100% sensitivity and specificity, the value for the area under the ROC curve is 1.0. It is important to note that the LDA, like the parameters calculation, was also implemented through a software developed with Matlab language.
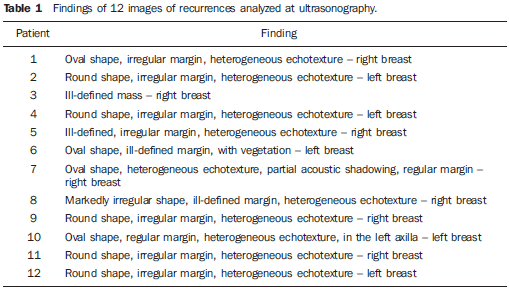
RESULTS Local tumor recurrences at ultrasonography Ultrasonography of the 12 patients with local recurrence demonstrated the following findings: 10 patients presented nodule, two presented mass, and one of them presented microcalcifications in association with nodule. The recurrences of nodules and masses were analyzed at ultrasonography demonstrating a prevalence of heterogeneous echotexture (Table 1). Examples of these lesions are shown on Figure 1. Regarding the nodules dimensions, the values ranged from 1.5 cm to 2.5 cm.
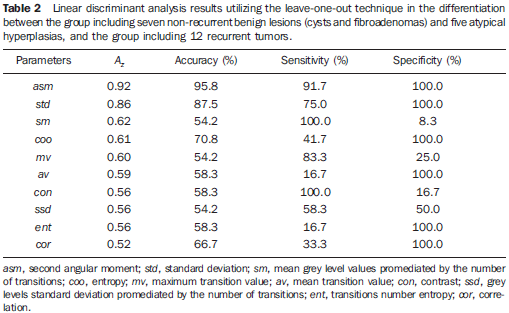
Ultrasonography of non-recurrent lesions Among the 24 patients who did not present recurrence in the surgical site, three presented malignant nodular lesion, with hypoechogenicity and irregular margins. Among the remaining 21 images, 7 presented benign nodules (3 cysts and 4 fibroadenomas), 5 atypical non-nodular hyperplasias, and 9, fibrocystic alterations. The cysts presented nodular shape, thin walls and posterior shadowing. The fibroadenomas were markedly hypoechogenic, with slightly lobulated margins and predominant transverse diameter. On the other hand, the hyperplasias appeared as small, ill-defined nodules of approximately 0.5 cm and also hypoechogenic. Examples of these two lesions are shown on Figure 2. Considering that, besides the personal factor of the patients involved, atypical hyperplasias represent a risk factor for breast cancer(2-5), the authors have opted for developing a separate study about the texture parameters performance in the differentiation between hyperplasias and benign nodules, as described below. Texture parameters performance in the differentiation of lesions Aiming at evaluating the texture characteristics of breast lesions on sonographic images of patients submitted to breast-conserving surgery either with or without tumor recurrence, the texture parameters based on the GLCM and CC were submitted to linear discriminant analysis and evaluated in two different circumstances. The first one corresponded to the evaluation of the parameters capacity of differentiating the group including seven non-recurrent benign lesions (cysts and fibroadenomas) and five atypical hyperplasias from a group including 12 tumor recurrences. In this analysis, the highest Az value (0.92) was obtained by the asm parameter of GLCM, while the cor parameter of GLCM presented the worst performance (Az = 0.52). In terms of accuracy, the best performance was achieved by asm, with sensitivity and specificity values > 91% (Table 2). The second best performance was achieved by the GLCM std, with accuracy and specificity values > 87% (Table 2).
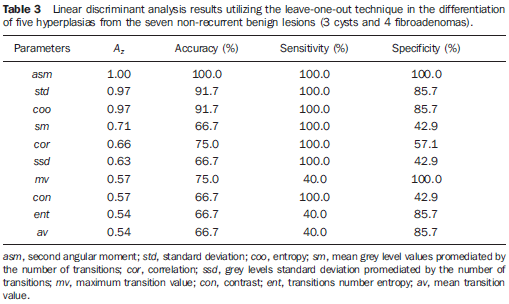
In a second moment, the authors evaluated the parameters performance in the differentiation between the 5 hyperplasias and the 7 non-recurrent benign lesions (3 cysts and 4 fibroadenomas). In this analysis, the asm parameter presented the best performance again, and could effectively differentiate hyperplasias from benign lesions (Table 3). Similarly, the second best parameter was std, with accuracy and sensitivity values > 91%.
The scatterplot on Figure 3A clearly shows the differentiation between the group of recurrent lesions and the group including benign lesions + hyperplasias (arrow on Figure 3A). The differentiation between benign lesions and hyperplasias is shown on Figure 3B. In this case, overlapping was not observed between the groups analyzed.
DISCUSSION Discussion of the texture parameters involved in the differentiation among lesions from different clinical origins Among the texture parameters utilized for differentiating benign lesions (cysts and fibroadenomas), atypical hyperplasias and tumor recurrences, the asm calculated from the GLCM presented the best performance. By definition, asm provides a measurement of the homogeneity degree of a determined texture, the more homogeneous being the texture, the higher the asm value. So, benign lesions that tend to present a more organized texture pattern, present higher asm values than those found among hyperplasias and tumor recurrences, respectively. This observation is compatible with the sonographic findings where most of times, the tumor recurrences presented heterogeneous texture (Table 1), while the benign lesions and hyperplasias presented hypoechogenicity and predominantly homogeneous texture. This is a relevant finding, considering that the quantitative analysis of complex or hypoechoic echotextures may present a low positive predictive value(26,27), so emphasizing the relevance of the quantification. Despite the reduced number of cases, the results of the present study can be considered as encouraging, suggesting that the utilization of quantitative texture parameters may be useful in the differentiation of benign lesions, atypical hyperplasias and malignant lesions related to tumor recurrence. Alvarenga et al.(20) had already demonstrated the texture parameters capacity to differentiate malignant and benign breast lesions, although not discussing the clinical origin of the lesions. Considering this method applicability, it may be adapted to run with other image acquisition techniques such as elastography, for example, since the image texture parameter tends to change under compression of the area of interest(28). Additionally, the present texture parameters may be combined with further quantitative parameters which represent other characteristics of lesions such as shape and contour(29), or even the vascular resistance index obtained by means of Doppler ultrasound (30). Thus, based on the methods developed by Alvarenga et al.(20,29), and on the results of the present study, it is expected that a system to aid in the diagnosis be developed in the future, contributing to reduce the subjectivity in the analysis of sonographic images and, consequently, reducing the intra- and interobserver variability observed in the clinical routine(31,32). It is important to note that texture parameters may be useful in the differentiation between benign and suspicious or malignant lesions, but the knowledge on the origin of the lesion, whether recurrent or not, depends on the knowledge about the clinical history of the patient. Thus, this aspect is discussed in the following topic. Supplementary discussion of clinical findings As already mentioned, the present study involved the evaluation of patients with tumor recurrence in the site of breast-conserving surgery and patients without recurrence in the surgical site. Among the patients with local recurrence, the youngest patient and with longer time interval (eight years) presented metastatic lesion in the ipsilateral axilla. The literature reports that unilateral and ipsilateral lymphadenomegaly tend to be metastatic lesions(2,5,7). However, according to Ikeda(2), the appearance of lesions six or more years after a breast-conserving surgery tend to represent a second primary tumor. The patient with shorter time interval (one year) was a menopausal woman and probably the cause for recurrence was the positive surgical margins. Among the patients who did not present local recurrence, presenting lesion in the contralateral breast, one of them had in situ carcinoma with areas of comedocarcinoma and probably multicentric lesions. Such types of lesions tend to involve different breast quadrants(11). In the present study, two of the patients without recurrence presented tumors in the contralateral breast which actually were asynchronous tumors with features different from the ones of the primary tumors. Atypical ductal hyperplasia increases four to five times the risk of breast cancer in the general population(2). Histological study did not demonstrate atypical lobular hyperplasia that would increase the risk of in situ lobular carcinoma(2). As a proliferative disease, hyperplasia presents a risk based on its cellularity (33), mainly atypical ductal hyperplasia and lobular hyperplasia, the latter considered as a higher risk factor(2,4,6). Probably, benign lesions in the contralateral breast were already present at the moment of the primary lesion diagnosis.
CONCLUSION The performance of the computer-aided method for quantifying breast lesions texture on sonographic images was evaluated in two groups of patients with different clinical nature as follows: patients submitted to breast-conserving surgery, either with or without tumor recurrence. With the asm parameter of GLCM, the authors could differentiate benign lesions, atypical hyperplasias and recurrent malignant lesions based on their texture features. Despite the limited number of images, the present results may be considered as encouraging, considering that in the future development of a CAD system, an association of this parameter with the knowledge of the patient's clinical history may aid in the definition of the diagnosis and prognosis of the tumor recurrence.
REFERENCES 1. Orel SG, Troupin RH, Patterson EA, et al. Breast cancer recurrence after lumpectomy and irradiation: role of mammography in detection. Radiology. 1992;183:201-6. [ ] 2. Ikeda DM. Breast imaging: the requisites. 1st ed. Philadelphia: Elsevier Mosby; 2004. [ ] 3. Birdwell RL. Pocket radiologist. Breast top 100 diagnoses. 1st ed. Salt Lake City: Amirsys; 2003. [ ] 4. Copeland EM. Special problems related to the operative site: local recurrence, the augmented breast and the contralateral breast. In: Bland KI, Copeland EM, editors. The breast: comprehensive management of benign and malignant diseases. 2nd ed. Philadelphia: WB Saunders; 1991. p. 1012-20. [ ] 5. Simmons RM, Osborne MP. Treatment of recurrent ductal carcinoma in situ. In: Silverstein MJ, editor. Ductal carcinoma in situ of the breast. Baltimore: Williams & Wilkins; 1997. p. 569-75. [ ] 6. Clifford AH, Larry N. Systemic treatment of stage I breast cancer. In: Roses DF, editor. Breast cancer. 1st ed. Philadelphia: Churchill Livingstone; 1999. p. 417-41. [ ] 7. Stevens RE, Cooper JS. Radiotherapy for in situ, stage I and stage II breast cancer. In: Roses DF, editor. Breast cancer. 1st ed. Philadelphia: Churchill Livingstone; 1999. p. 385-415. [ ] 8. Pressman PI. Treatment of bilateral breast cancer. In: Roses DF, editor. Breast cancer. 1st ed. Philadelphia: Churchill Livingstone; 1999. p. 483-91. [ ] 9. Wallgren A, Bonetti M, Gelber RD, et al. Risk factors for locoregional recurrence among breast cancer patients: results from International Breast Cancer Study Group Trials I through VII. J Clin Oncol. 2003;21:1205-13. [ ] 10. Katz A, Strom EA, Buchholz TA, et al. Locoregional recurrence patterns after mastectomy and doxorubicin-based chemotherapy: implications for postoperative irradiation. J Clin Oncol. 2000; 18:2817-27. [ ] 11. Philpotts LE, Lee CH, Haffty BG, et al. Mammographic findings of recurrent breast cancer after lumpectomy and radiation therapy: comparison with the primary tumor. Radiology. 1996;201:767-71. [ ] 12. Rissanen TJ, Mäkäräinen HP, Mattila SI, et al. Breast cancer recurrence after mastectomy: diagnosis with mammography and US. Radiology. 1993;188:463-7. [ ] 13. Goldberg V, Manduca A, Ewert DL, et al. Improvement in specificity of ultrasonography for diagnosis of breast tumors by means of artificial intelligence. Med Phys. 1992;19:1475-81. [ ] 14. Garra BS, Krasner BH, Horii SC, et al. Improving the distinction between benign and malignant breast lesions: the value of sonographic texture analysis. Ultrason Imaging. 1993;15:267-85. [ ] 15. Lefebvre F, Meunier M, Thibault F, et al. Computerized ultrasound B-scan characterization of breast nodules. Ultrasound Med Biol. 2000;26:1421-8. [ ] 16. Sivaramakrishna R, Powell KA, Lieber ML, et al. Texture analysis of lesions in breast ultrasound images. Comput Med Imaging Graph. 2002;26:303-7. [ ] 17. Kuo WJ, Chang RF, Lee CC, et al. Retrieval technique for the diagnosis of solid breast tumors on sonogram. Ultrasound Med Biol. 2002;28:903-9. [ ] 18. Chen DR, Chang RF, Huang YL. Breast cancer diagnosis using self-organizing map for sonography. Ultrasound Med Biol. 2000;26:405-11. [ ] 19. Chang RF, Wu WJ, Moon WK, et al. Improvement in breast tumor discrimination by support vector machines and speckle-emphasis texture analysis. Ultrasound Med Biol. 2003;29:679-86. [ ] 20. Alvarenga AV, Pereira WCA, Infantosi AFC, et al. Complexity curve and grey level co-occurrence matrix in the texture evaluation of breast tumor on ultrasound images. Med Phys. 2007;34:379-87. [ ] 21. Al-Janobi A. Performance evaluation of cross-diagonal texture matrix method of texture analysis. Pattern Recogn. 2001;34:171-80. [ ] 22. Baheerathan S, Albregtsen F, Danielsen HE. New texture features based on the complexity curve. Pattern Recognition. 1999;32:605-18. [ ] 23. Johnson RA, Wichern DW. Applied multivariate statistical analysis. 4th ed. Upper Saddle River: Prentice-Hall; 1998. [ ] 24. Bishop CM. Neural networks for pattern recognition. Oxford: Clarendon Press; 1995. [ ] 25. Metz CE. ROC methodology in radiologic imaging. Invest Radiol. 1986;21:720-33. [ ] 26. Nascimento JHR, Silva VD, Maciel AC. Acurácia dos achados ultrassonográficos do câncer de mama: correlação da classificação BI-RADS® e achados histológicos. Radiol Bras. 2009;42:235-40. [ ] 27. Calas MJG, Koch HA, Dutra MVP. Ultra-sonografia mamária: avaliação dos critérios ecográficos na diferenciação das lesões mamárias. Radiol Bras. 2007;40:1-7. [ ] 28. Fleury EFC, Rinaldi JF, Piato S, et al. Apresentação das lesões mamárias císticas à ultra-sonografia utilizando a elastografia. Radiol Bras. 2008; 41:167-72. [ ] 29. Alvarenga AV, Infantosi AFC, Pereira WCA, et al. Assessing the performance of the normalised radial length and convex polygons in distinguishing breast tumours on ultrasound images. Rev Bras Eng Biomed. 2006;22:181-9. [ ] 30. Schmillevitch J, Guimarães Filho HA, De Nicola H, et al. Utilização do índice de resistência vascular na diferenciação entre nódulos mamários benignos e malignos. Radiol Bras. 2009;42:241-4. [ ] 31. Calas MJG, Almeida RMVR, Gutfilen B, et al. Intraobserver interpretation of breast ultrasonography following the BI-RADS classification. Eur J Radiol. 2009 May 5. [Epub ahead print] [ ]. 32. Kestelman FP, Souza GA, Thuler LC, et al. Breast Imaging Reporting and Data System - BIRADS®: valor preditivo positivo das categorias 3, 4 e 5. Revisão sistemática da literatura. Radiol Bras. 2007;40:173-7. [ ] 33. Pandey S, Kornstein MJ, Shank W, et al. Columnar cell lesions of the breast: mammographic findings with histopathologic correlation. Radiographics. 2007;27 Suppl 1:S79-89. [ ] Received December 22, 2008. * Study developed at COPPE/UFRJ - Instituto Alberto Luiz Coimbra de Pós-Graduação e Pesquisa de Engenharia, Programa de Engenharia Biomédica, Rio de Janeiro, RJ, Brazil. Financial support: Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (Capes) and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq). |
|
Av. Paulista, 37 - 7° andar - Conj. 71 - CEP 01311-902 - São Paulo - SP - Brazil - Phone: (11) 3372-4544 - Fax: (11) 3372-4554