Radiologia Brasileira - Publicação Científica Oficial do Colégio Brasileiro de Radiologia
AMB - Associação Médica Brasileira CNA - Comissão Nacional de Acreditação
 Vol. 42 nº 5 - Sep. / Oct. of 2009
Vol. 42 nº 5 - Sep. / Oct. of 2009
|
ORIGINAL ARTICLE
|
|
Evaluation of contrast media submitted to ionizing radiation |
|
|
Autho(rs): Kátia Elisa Prus Pinho, Pedro Miguel Gewehr, Caroline Werner Pereira da Silva, Andersson Barison, João Gilberto Tilly Júnior, Danyel Scheidegger Soboll |
|
|
Keywords: Imaging diagnosis, Contrast media, Ionizing radiation, Nuclear magnetic resonance spectroscopy, X-rays, Gamma rays |
|
|
Abstract:
IM. Sc., Academic Extension Coordinator and Professor, Course of Technology in Radiology, Universidade Tecnológica Federal do Paraná (UTFPR), Curitiba, PR, Brazil
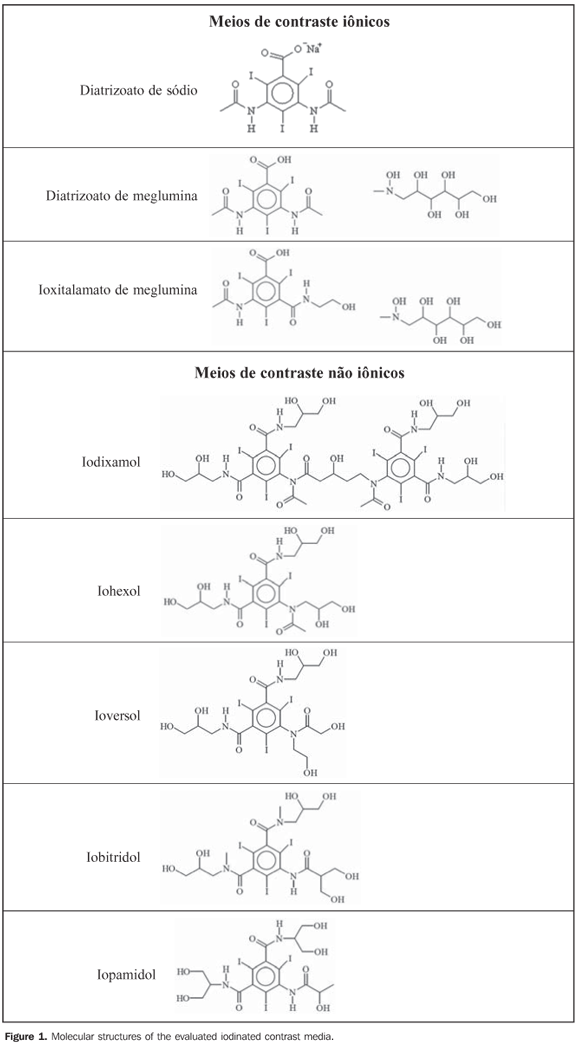
INTRODUCTION Radiologic contrast media are compounds which are introduced into the organism by different vias, in order to increase in the definition of radiographic images due to the contrast improvement caused by such compounds, allowing acquisition of high-definition images and as consequence higher accuracy in diagnostic imaging(1,2). The use of compounds to improve the quality of radiologic images dates more than half a century(3). Since then, adverse reactions resulting from the oral or intravenous administration of a foreign substance to the human body have been reported as such compounds are not always harmless, and may alter the blood circulation causing unexpected reactions(4,5). Taking this fact into account, several precautions must be taken with patients as well as in the preparation and storage of contrast media(6,7). All iodinated contrast media currently in use are derived from 2,4,6-triiodobenzoic acid (Figure 1). They are classified according to their physicochemical characteristics, including chemical structure, osmolarity, viscosity, number of iodine atoms in the structure, biological properties, ionization capacity in solution, hydrosolubility, lipophilicity and toxicity (6). Ionic contrast media are those capable of dissociating into cations and anions in aqueous solutions, while the non-ionic ones do not dissociate (Figure 1), but interact with water molecules by means of intermolecular interactions(5). Special care in the storage and asepsis of contrast media is essential, including storage away from light, as they are photosensitive, and away from the incidence of x-rays due to the possibility of ionizing radiations causing molecular degradation, thus changing the molecular structure of the contrast media and, as a consequence, its contrast properties on the radiological images. Additionally, it is important to store them under temperatures between 15 and 25ºC, as in lower temperatures could occurs the crystallization of the contrast media, checking storage time and not using vials after they have been opened for more than 24 hours, due to the risk of contamination by microorganisms(8).
The present study investigates the influence of from x-rays and gamma rays ionizing radiations on the molecular structure stability of several commercial contrast media widely employed in radiologic imaging diagnosis by radiography and computed tomography by means of 1H and 13C nuclear magnetic resonance (NMR) spectroscopy. The aim of the work consisted in checking possible changes on the molecular structure of the contrast media due to the incidence of ionizing radiations, once they would alters the physicochemical and biological properties, as well as their toxicity, in order to corroborate with the reported data in the literature(8).
MATERIALS AND METHODS Samples Eight different iodinated contrast media, three ionic and five non-ionic (Figure 1) were obtained from four manufacturers available in Brazil. The contrast media, with respective manufacturers were as follow: Iopamiron 300 and Pielograf 76% (Bayer Schering Pharma; Berlin, Germany), Ominipaque and Visipaque (Farmasa; São Paulo, Brazil), Henetix and Telebrix (Guerbet; Paris, France), Optiray 320 and Optiray 350 (Mallinckrodt; Saint Louis, USA). Eight aliquots were taken of each contrast medium, of which four were submitted to x-rays radiation exposition, two to gammas ray radiation exposition and two were kept as control, without any radiation exposure. In order to irradiate the contrast media, aliquots of 1.7 mL of each medium were transferred in to microcentrifuge tubes, identified by letters in order to make impossible to the instrument operator to known the samples origin. The sample preparation was performed in the Chemistry Laboratory of the Universidade Tecnológica Federal do Paraná (UTFPR), according to asepsis procedure for each open and aspirated vial(9). X-rays irradiation The samples irradiation by x-rays was performed in the Radiotherapy Department of Hospital Erasto Gaertner in the city of Curitiba, PR, Brazil, on a Radcal 9010 radiation monitor and a 10X5-6 Radcal ionization chamber (Radcal Corp.; Monrovia USA), with a sensitive volume of 6 cm> calibrated to the radiodiagnosis energy level, thus determining the exact radiation that the samples were being exposed. The source of radiation utilized was an x-ray tube from a RMX 625 R radiotherapy simulator (Raytheon Medical Systems; Melrose Park, USA), with inherent filtration equivalent to 0.5 mm of aluminum. The samples were exposed to different doses of x-rays radiation, ranging from 9.85 to 10200 mR (Table 1), at a distance of 50 cm from the source, and voltage of 90 kV(10).
Gamma rays irradiation The irradiation of the samples by gamma rays was performed using a mean energy of 1.25 MeV, from a cobalt-60 source installed in a Theratron 780 C teletherapy unit (MDS Nordion; Ontario, Canada) with a 0.5 cm bolus of gel over the samples. The source-surface distance was 50 cm and the performance at such distance was 5 Gy/min. The samples were exposed to two radiation doses: 0.1 and 10 Gy (10). In both the cases the samples were irradiated in an 8 × 8 cm2 field. NMR Analysis The 1H and 13C{1H} NMR spectra were acquired on an Avance 400 (Bruker; Karlsruhe, Germany) NMR spectrometer operating at 9.4 Tesla, installed in the NMR Laboratory of Universidade Federal do Paraná (UFPR), observing the 1H and 13C nuclei at 400.13 and 100.62 MHz, respectively, in D2O (deuterated water) at room temperature of approximately 295 K in a 5 mm direct detection multinuclear probe. For this, aliquots of 0.2 ml of each contrast medium were filtered in cotton directly into 5 mm NMR tubes, with the help of Pasteur pipettes, follow by 0.4 ml of D2O containing (3-trimethylsilyl)-2,2',3,3'-tetradeuteropropionic acid, sodium salt (TMSP-d4 – internal reference)(11). The 1H NMR spectra were acquired with the zg pulse sequence by accumulating four averages, 64 K points (1 K = 1024) and spectral window of ~13 ppm (11,12). In some samples it was necessary the pre-saturation of the water signal, using the zgpr pulse sequence. On their turn, the 13C{1H} NMR spectra were acquired with the zgpg30 pulse sequence, accumulating 1024 averages, 32 K points and spectral window of ~255 ppm. Both the 1H and 13C{1H} NMR spectra were processed with aid of TopSpin software (Bruker; Karlsruhe, Germany) by applying exponential multiplications of the free induction decay (FID) by factors of 0.3 and 3.0 Hz for the construction of 1H and 13C NMR spectra with 64 K and 32 K points, respectively. All NMR chemical shifts are giving in ppm related to the internal standard signal from TMSP-d4 at 0.0 ppm(11).
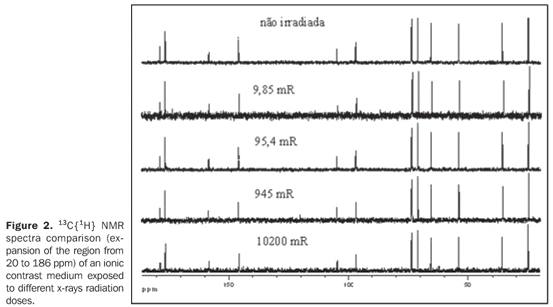
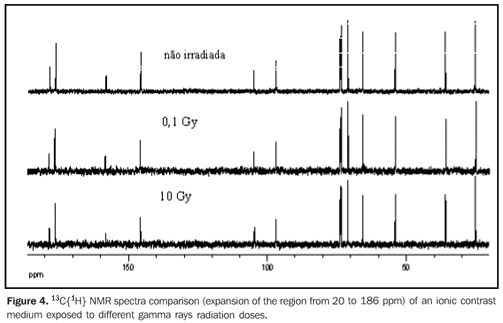
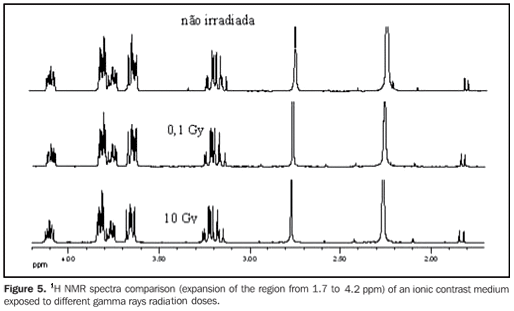
RESULTS In order to evaluating the effected of the application of ionizing radiations on contrast media utilized in radiology, 13C{1H} and 1H NMR spectra were acquired from the samples after being exposed to ionizing radiations from by x-rays or gamma rays. In a similar way, NMR spectra were obtained from samples of the same contrast media without exposition to ionizing radiations, used as references for comparison with those irradiated. Figure 2 and 3 show the 13C{1H} and 1H NMR spectra comparison, respectively of a ionic contrast medium sample that was exposed to different x-rays radiation doses, while Figures 4 and 5 show the 13C{1H} and 1H NMR spectra comparison, respectively of ionic contrast medium sample that was submitted to different gamma ray radiation doses.
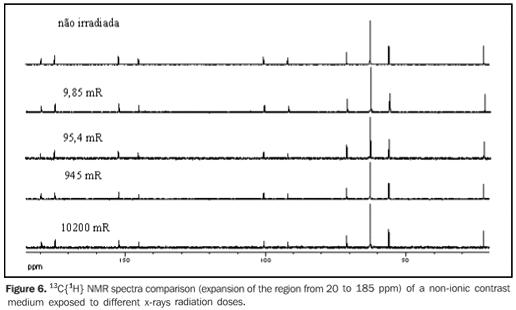
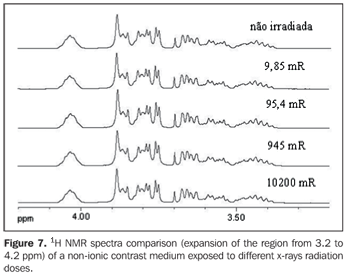
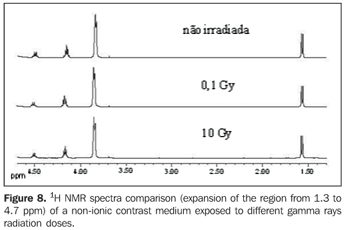
Figures 6 and 7 present the 13C{1H} and 1H NMR spectra comparison, respectively, of a non-ionic contrast medium sample submitted to different x-rays radiation doses, while Figure 8 shows the 1H NMR spectra comparison of non-ionic contrast medium sample submitted to different gamma ray radiation doses.
DISCUSSION The 13C{1H} NMR spectra in both ionic and non-ionic contrast media irradiated by x-rays or gamma rays showed that at the energy levels utilized any changes in the molecular structures of the investigated contrast media occurs, as observed on Figures 2, 4 and 6 for a determined contrast medium. In the same way, the 1H NMR spectra, which has higher sensitivity than those from 13C{1H}(11) , also showed no changes on the chemical structure of the investigated contrast media, as shown on Figures 3, 5, 7 and 8. In other words, there was no degradation of the analyzed contrast media, as there were no evidences of new signals in the NMR spectra, which would be an indicative of formation of new compounds, as a consequence of decomposition of the contrast media. This becomes evident when the NMR spectra of the control samples which were not submitted to any radiation were compared with those from samples submitted to irradiation, either by x-rays or gamma rays. In other words, as all samples presented the same spectral profile at the 1H and 13C{1H} NMR analysis (Figures 2 to 8). Therefore, the molecular structure of the investigated contrast media is not affected when exposed to irradiation with x-rays or gamma rays, and then there is no problem in storing them in the equipment room or close to the equipment in which the examinations are performed. Emphasis is given to the fact that the radiation received by the samples during the trials was direct, while in an actual study the radiation would be indirect and, therefore, the levels of radiation in such cases would be different from those utilized in the present study. In spite of the fact that some contrast media manufacturers recommend avoiding their storage in the presence of disperse ionizing radiations, the present study did not detect any influence of ionizing radiations from x-rays or gamma rays on the molecular structure of the evaluated iodinated contrast media in the described conditions. Probably, this observation from manufacturers refers as a general precaution in the sense of preserving and manipulating only the necessary quantities of contrast media for the day's examination needs, while greater quantities might, in some cases, be stored in the examination room being unnecessarily exposed and becoming a constant practice in diagnostic imaging centers.
CONCLUSION The investigations demonstrated that the ionizing radiation utilized in radiology diagnostic imaging by radiography and computed tomography do not cause any changes in the molecular structures (degradation) of the currently utilized contrast media, independently of exposure levels from x-rays or gamma rays radiation in which the contrast media were submitted, and thus allowing the clinical use of such contrast media. Acknowledgments The authors are grateful to the Companies Bayer Schering Pharma, Farmasa, Guerbet and Mallinckrodt, that kindly provided contrast media samples as well as to the Chemistry Laboratories of UTFPR and UFPR and to the Hospital Erasto Gaertner, for supporting the development of the present study with their equipments.
REFERENCES 1. Falgas BJ, Hurlé ADG, Lecumberri VN, et al. Farmacia hospitalaria. Madrid: Sociedad Española de Farmacia Hospitalar; 2002. [ ] 2. Bontrager LK. Tratado de técnica radiológica e base anatômica. Rio de Janeiro: Guanabara Koogan; 1999. [ ] 3. Bettmann MA. Frequently asked questions: iodinated contrast agents. Radiographics. 2004;24 Suppl 1:S3–10. [ ] 4. Asociación Argentina de Alergia e Imunología Clínica. Reacciones adversas a medios de contraste radiológicos: criterios y conductas. AAAelC y SAR. 2001;32:101–5. [ ] 5. Schild H. Todo sobre medios de contraste: ver o no ver. España: Schering AG; 1995. [ ] 6. Pinho KEP. Avaliação de fatores de riscos na utilização de contrastes iodados em exames de urografia excretora [dissertação de mestrado]. Curitiba: CPGEI/UTFPR; 2006. [ ] 7. Colégio Brasileiro de Radiologia. Assistência à vida em radiologia. São Paulo: CBR; 2000. [ ] 8. Sugawara AM, Daros KAC. Manual de meios de contraste em raios X. São Paulo: São Camilo; 2004. [ ] 9. Nischimura L, Potenza M, Cesaretti I. Enfermagem nas unidades de diagnóstico por imagem. São Paulo: Atheneu; 1999. [ ] 10. Stanton R, Stinson D. Applied physics for radiation oncology. Madison: Medical Physics Pub; 1996. [ ] 11. Günter H. NMR spectroscopy: basic principles, concepts, and applications in chemistry. Chichester: John Wiley & Sons; 1995. [ ] 12. Willard HH, Merritt LL Jr, Dean JA, et al. Instrumental methods of analysis. Belmont: Wadsworth Pub; 1988. [ ] Received May 21, 2009. Accepted after revision August 21, 2009. * Study developed at Universidade Tecnológica Federal do Paraná (UTFPR), in a partnership with the Laboratory of Nuclear Magnetic Resonance, Department of Chemistry – Universidade Federal do Paraná (UFPR) and Department of Radiotherapy – Hospital Erasto Gaertner, Curitiba, PR, Brazil. |
|
Av. Paulista, 37 - 7° andar - Conj. 71 - CEP 01311-902 - São Paulo - SP - Brazil - Phone: (11) 3372-4544 - Fax: (11) 3372-4554