Radiologia Brasileira - Publicação Científica Oficial do Colégio Brasileiro de Radiologia
AMB - Associação Médica Brasileira CNA - Comissão Nacional de Acreditação
 Vol. 42 nº 5 - Sep. / Oct. of 2009
Vol. 42 nº 5 - Sep. / Oct. of 2009
|
WHICH IS YOUR DIAGNOSIS?
|
|
Which is your diagnosis? |
|
|
Autho(rs): Marcelo Souto Nacif, Denise Castro de Souza Côrtes, Amarino Carvalho de Oliveira Junior, Luiz Carlos Simões, Ricardo Andrade Fernandes de Mello, Edson Marchiori |
|
|
IFellow PhD degree (Cardiac MRI), Teacher at Unifeso, Teresópolis, RJ, Brazil
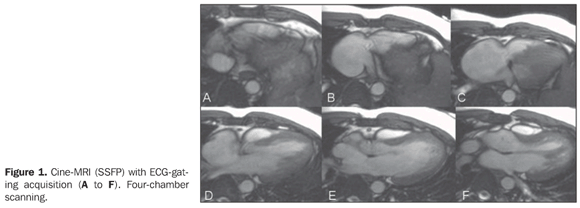
A male 29-year-old patient presenting right ventricular hypoplasia. No therapeutic approach was reported. The patient was poorly symptomatic, with a normal physical development, with no limitation to daily activities. Routine examinations identified high suspicion of right–left shunt. Transthoracic study by two experienced physicians (more than 20-year experience) had identified right ventricular hypoplasia and pulmonary stenosis. However, the diagnosis was doubtful in relation to right–left shunt. The patient was referred to the Unit of Radiology and Imaging Diagnosis of Hospital Pró-Cardíaco for investigation of interatrial septum and shunt. Images description Figure 1. Cine-MRI (SSFP) with ECG-gating acquisition (A to F). Four-chamber scanning. Note the reduced size of the right cavity and the defect of the interatrial septum adjacent to the superior vena cava entry into the right atrium.
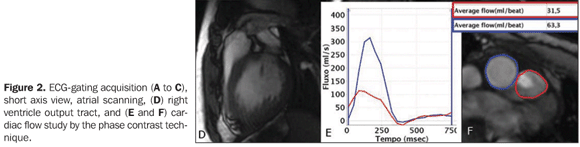
Figure 2. ECG-gating acquisition (A to C), short axis view, atrial scanning, (D) right ventricle output tract, and (E and F) cardiac flow study by the phase contrast technique. On images A to C, note the absence of a septum at the upper portion, measuring 1.5 cm. On D, pulmonary supravalvar stenosis is identified, with a visible anterograde flush. On E and F, flow study demonstrates that the mean velocity in the pulmonary trunk 31.5 ml and 63.3 ml in the aorta, compatible with Qs/Qp = 2.0 and right–left shunt.
Diagnosis: Right ventricular hypoplasia associated with interatrial communication and pulmonary supravalvar stenosis in an adult patient.
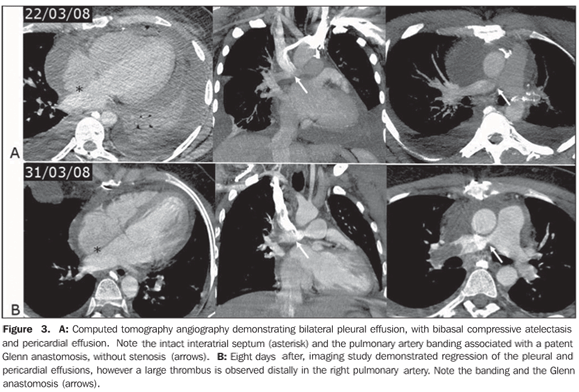
COMMENTS The number of patients with congenital heart disease (CHD) who grow to adulthood has gradually increased. Many of these adult individuals present difficulties in the clinical and surgical approach. A retrospective study developed in the period from 1980 to 2000 followed-up 206 adult patients aged between 18 and 71 years, with CHD, who were admitted to a tertiary hospital. The most frequently found congenital anomaly was interatrial communication (53%), followed by ventricular septal defect (interventricular communication) (11%) and Fallot's tetralogy (11%). Many patients were asymptomatic, and the most frequent complaints were dyspnea and fatigue. Cyanosis was present in 27 patients (13%), and 17 of them (63%) underwent surgical correction. Surgical mortality and intra-hospital complications were slightly more significant in adult individuals than those reported in children with similar cardiac lesions(1–3). Transthoracic echocardiography (TTE) is a study performed on a routine basis to evaluate heart diseases. However, the limitations of this method are widely known, one of them is the limited study window. In a significant number of cases of congenital disease, especially in the assessment of adult patients and in the identification of shunts, TTE does not settle a diagnosis, with a high number of false-negative results being observed. The clinical suspicion plays a significant role in the definition of the diagnosis and, currently, cardiologists and clinicians in this area indicate transesophageal echocardiography (TEE) for the diagnosis(3). With the technological developments and the introduction of new radiologic devices, imaging methods such as cardiac computed tomography (CT) and cardiac magnetic resonance imaging (MRI) allow the noninvasive diagnosis of morphological and anatomical alterations. In the case of congenital cardiopathies, magnetic resonance imaging can provide hemodynamic data with cine-MRI and phase-contrast (PC) techniques that, if appropriately applied, can be extremely useful for the diagnosis(4,5). Atrial septal defect and interatrial communication Atrial septal defect is the most frequent congenital cardiopathy, with an incidence of one per 1,500 live newborns(5). This cardiopathy is commonly subdivided into five presentations: 1 – the most frequent one is known as patent foramen ovale, where the typical closure by the septum primum does not occur after birth, in opposition to the superior limbic band; 2 – the ostium secundum is the second most frequent presentation and involves the fossa ovalis, usually related to a defect in the closure of the septum primum; it may be single or multiple, in association with several fenestrations; 3 – the third most common presentation is known as ostium primum and occurs in the lower third of the inferior interatrial septum, below the fossa ovalis and above the malformed leaflets of the atrioventricular valves, i.e., it may be associated with a cleft in the anterior leaflet of the mitral valve; 4 – the least frequent one is the venous sinus type that may be adjacent to the superior vena cava entry, or inferiorly, adjacent to the interatrial septum. It is important to note that the venous sinus type of interatrial defects generally are found between the pulmonary vein of the right upper lobe and the superior vena cava entry; in these cases, the defect is associated with an anomalous pulmonary venous return, involving the pulmonary vein of the right upper lobe and that may be connected to the superior vena cava itself or to the azygos vein. However, these defects may involve any region adjacent to the veins of the right middle and lower lobes; 5 – the coronary sinus type atrial septal defect is the least frequently found, and occurs as this portion of the septum is partially or completely unroofed, allowing the passage of the flow through the sinus. In these cases, one must be attentive to the diagnosis of persistent left superior vena cava that apparently is connected to the left atrium (Raghib's syndrome)(2,3,6). The left–right shunt associated with the presence of atrial septal defect leads to an increase in the volume of the right cavities, with ectasia of the pulmonary trunk and arteries. Along years, an undiagnosed defect induces to pulmonary arterial hypertension, with parietal stiffening of the pulmonary arteries in about 5–10% of cases, with possibility of shunt reversal (right–left) as a result of the increase in the pressure in the right cavity (Eisenmenger's syndrome)(3). The role of cardiac MRI Because of its multiplanar capacity and ability for anatomic, functional and flow evaluation, the latest, specifically, by the PC technique, MRI is a noninvasive, clear and objective method for the diagnosis of either suspected or unknown CHD in adolescent and adult individuals(5). With the nationwide availability of the method, it can be utilized as a substitute for TEE in some cases. Cardiac MRI has been used in the planning of surgical or percutaneous correction of atrial septal defects. Durongpisitkul et al.(7) have demonstrated that cardiac MRI may be useful in the measurement of the largest diameter of the atrial septal defect and of its postero-inferior rim, with data similar to those of cardiac catheterization, which anatomically indicates the percutaneous closure. Frequently, TTE fails to detect some atrial septal defects; in these cases, cardiac MRI plays an alternative and differentiated role in the characterization of these rare atrial shunts, typically represented by lesions such as septal defect of venous sinus type, anomalous pulmonary venous return, and septal defect of coronary sinus type(5,8). The pulmonary flow/systemic flow (Qp/Qs) ratio can be determined by cine-PC, PC technique, and through the comparison of the contraction (systolic) volume in the volume measurements between the right and left ventricles, and through the correct measurement of pulmonary artery and aorta ratios. The Qp/Qs ratio is closely correlated with the oxymetry performed during the catheterization(5,8,9). In practice, as an atrial septal defect is found, data indicating the necessity of this defect closure must be informed. Additionally to symptoms, data regarding left–right shunt are required. The data which corroborate the necessity of closure are: diameter > 5 mm, right ventricle dilatation; interventricular septum rectification at diastole (caused by the hypertension secondary to hyperflow); Qp/Qs > 1.5–2.0 and associated cardiac or vascular abnormalities(8). Based on the above considerations, one can note that an accurate protocol is re quired for evaluation of these patients, with long-axis, four-chamber and short-axis scanning of the whole atrial septum, two-axis PC study perpendicularly to the septum, besides the study of the pulmonary artery and aorta, in association with three-dimensional angio-MRI, T1-weighted sequence with gadolinium. Even with these specific protocols, there is a possibility of a dubious diagnosis in cases of septum primum defect, overestimation of the interatrial defect size, false-positive diagnosis, and failure in the anatomical quantification of multifenestrated defects. In the present case, the preoperative evaluation by cardiac MRI demonstrated right ventricle hypoplasia, venous sinus type interatrial communication with right-left shunt, Qs/Qp = 2.0, pulmonary supravalvar stenosis and mild dysfunction of the left ventricle, 45% ejection fraction. Surgery and postoperative follow-up In the present case, the patient was preoperatively submitted to bleeding until hematocrit levels reached 50%. The surgical correction was done under extracorpo real circulation, involving atrioseptoplasty with bovine pericardium patch, pulmonary stenosis repair and anastomosis of the superior vena cava with the right branch of the pulmonary artery (Glenn procedure), with this pulmonary branch banding. The patient presented a typical peroperative bleeding and hemoderivative replacement was not required. The progression in the immediate postoperative period was satisfactory, with good arterial saturation and normal drainage. The patient was extubated few hours after the surgery. The patient remained under full anticoagulation therapy after the drain tubes removal, progressing with mild pericardial effusion, the full heparin anticoagulation being suspended. However, the patient developed pleural-pericardial effusion, requiring surgical drainage, and was treated with a single daily dose of enoxiparin and low-dose corticoid (Figure 3A). Ventilation discomfort with hypoxemia was observed after the effusions regression. The patient was submitted to CT-angiography that identified thrombosis in the right pulmonary artery branch, posteriorly to the anastomosis with the superior vena cava that was patent (Figure 3B).
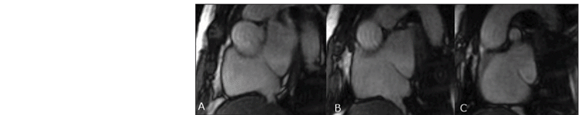
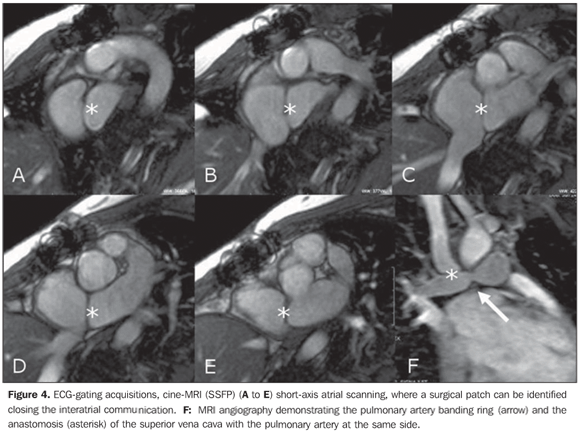
Intravenous heparin therapy was restarted, with a strict monitoring of the thromboplastin time, in association with antiplatelet aggregation by means of a 100 mg aspirin daily dose, with a good response. Later, oral coumarin anticoagulation was introduced, maintaining the association with acetylsalicylic acid. The follow-up with angiotomography demonstrated a complete thrombi resolution and recanalization of the pulmonary arterial tree. Currently, the patient is under follow-up on an outpatient basis; is asymptomatic, with 94% room air arterial saturation, 47% hematocrit level, using anticoagulation and antiplatelet aggregation agents and undergoing a program of cardiac rehabilitation. The postoperative cardiac MRI (Figure 4) allowed the observation of the closure of the interatrial septum and of the sys temic/pulmonary venous Glenn's shunt, with a permeable anastomosis and with no alteration.
Final considerations Cardiac MRI will be more and more available for the cardiological practice and has already become a relevant tool in the approach of patients with congenital cardiopathy, particularly in the evaluation of shunts that otherwise would hardly be evaluated by TTE. In many cases, this method will substitute the TEE in the diagnosis of such cardiopathy.
REFERENCES 1. Hannoush H, Tamim H, Younes H, et al. Patterns of congenital heart disease in unoperated adults: a 20-year experience in a developing country. Clin Cardiol. 2004;27:236–40. [ ] 2. Abbara S, Walker TG. Diagnostic imaging: cardiovascular. Manitoba: Amirsys; 2008. [ ] 3. Webb GD, Smallhorn JF, Therrien J, et al. Doença cardíaca congênita. In: Zipes DP, Libby P, Bonow RO, et al., editores. Braunwald–Tratado de doenças cardiovasculares. 7ª ed. Rio de Janeiro: Elsevier; 2006. p. 1489–552. [ ] 4. Grizzard JD, Judd RM, Kim RJ. Hemodynamic assessment and congenital heart disease. In: Grizzard JD, Judd RM, Kim RJ, editors. Cardiovascular MRI in practice – a teaching file approach. London: Springer-Verlag; 2008. p. 42–8. [ ] 5. Valente AM, Powell AJ. Clinical applications of cardiovascular magnetic resonance in congenital heart disease. Magn Reson Imaging Clin N Am. 2007;15:565–77, vi. [ ] 6. Campbell M. Natural history of atrial septal defect. Br Heart J. 1970;32:820–6. [ ] 7. Durongpisitkul K, Tang NL, Soongswang J, et al. Predictors of successful transcatheter closure of atrial septal defect by cardiac magnetic resonance imaging. Pediatr Cardiol. 2004;25:124–30. [ ] 8. Wald RM, Powell AJ. Congenital heart disease – indications, patient preparation and simple lesions. In: Kwong RY, editor. Cardiovascular magnetic resonance imaging. Totowa: Humana Press; 2008. p. 537–65. [ ] 9. Powell AJ, Tsai-Goodman B, Prakash A, et al. Comparison between phase-velocity cine magnetic resonance imaging and invasive oximetry for quantification of atrial shunts. Am J Cardiol. 2003;91:1523–5, A9. [ ] Study developed at Unit of Radiology and Imaging Diagnosis – Hospital Pró-Cardíaco, and at Department of Radiology – Universidade Federal do Rio de Janeiro (UFRJ), Rio de Janeiro, RJ, Brazil. |
|
Av. Paulista, 37 - 7° andar - Conj. 71 - CEP 01311-902 - São Paulo - SP - Brazil - Phone: (11) 3372-4544 - Fax: (11) 3372-4554