Radiologia Brasileira - Publicação Científica Oficial do Colégio Brasileiro de Radiologia
AMB - Associação Médica Brasileira CNA - Comissão Nacional de Acreditação
 Vol. 42 nº 4 - July / Aug. of 2009
Vol. 42 nº 4 - July / Aug. of 2009
|
ORIGINAL ARTICLE
|
|
Utilization of vascular resistance index in the differentiation between benign and malignant breast nodules |
|
|
Autho(rs): Joel Schmillevitch, Hélio Antonio Guimarães Filho, Harley De Nicola, Ana Cheila Gorski |
|
|
Keywords: Breast, Breast lesions, Ultrasonography, Doppler, Resistance index |
|
|
Abstract:
ISpecialists in Radiology and Imaging Diagnosis, Directors for Centro Diagnóstico Schmillevitch, São Paulo, SP, Brazil
INTRODUCTION Ultrasonography is currently considered as the main adjuvant to mammography in the screening for malignant breast nodules. Main indications include the evaluation of circumscribed lesions visible at mammography, evaluation of palpable nodules without mammographic expression, as an aid in the diagnosis of focal asymmetries, and as a method of screening patients with increased breast density in the search for occult lesions. Additionally, it represents a relevant adjuvant method in invasive diagnostic procedures, such as biopsies and preoperative punctures(1-4). Gray scale characteristics have been widely accepted by the international literature as a sonographic criterion for differentiating malignant from benign breast lesions, since the publication of the study developed by Stavros et al.(5). However, a consensus is still to be reached about the utilization of Doppler in the diagnosis of breast diseases. Several studies have adopted qualitative and quantitative criteria in an attempt to distinguish malignant from benign breast lesions and also to predict the disease prognosis(6-15). The results achieved by these studies are unlike each other and, for this reason the Doppler utility in the diagnosis of breast cancer is still to be defined. For the purpose of aiding in the definition of the role of Doppler in the diagnosis of breast lesions, the authors present the results of a study aimed at evaluating the utility of vascular resistance índex values in the differentiation between malignant and benign breast nodules.
MATERIALS AND METHODS The present prospective study involved 37 female patients evaluated in the period between January and July 2006. All the patients participating in the study have signed a term of free and informed consent. The inclusion criterion was the presence of breast nodules > 1 cm sonographically visualized and classified into BI-RADS® categories III, IV or V for ultrasonography. These patients were submitted core needle biopsy for histological diagnosis, resulting in 37 biopsied nodules. The patients' ages ranged between 20 and 93 years. All the echographic examinations were performed previously to the invasive procedure. Both procedures were performed by a single, experienced investigator. The lesions were evaluated through the grayscale and subsequently by amplitude Doppler ultrasound. The color box was adjusted to include the lesion and also a small margin of the adjacent healthy breast tissue. The Doppler study was considered as positive in cases where at least one vessel was detected within or adjacent to the lesion, demonstrating an arterial flow pattern at the spectral analysis. In this case, pulsed Doppler was performed to evaluate the flow velocity, invariably in the most calibrous vessel, with subsequent calculation of the vascular resistance index (RI). Adjustments in pulse repetition frequency, gain, wall filter, and sample volume depth were made as necessary to improve de image, minimizing artifacts. The specimens for anatomopathological study were obtained through ultrasonography-guided core needle biopsy performed in all of the nodules, by the same investigator, in all the cases evaluated in the present study. Three to six fragments were collected from each solid nodule identified, with 14 or 16 Gauge needle and an automatic pistol device. The histological samples were fixed in buffer formaldehyde (10%) and subsequently sent to two pathologists specialized in breast diseases, who performed a joint analysis of all the cases. All of the nodules included in the present study were greater than 1.0 cm in diameter. For the purposes of statistical analysis, the RI values for the 37 patients were recorded on an Excel 2003 worksheet and exported to the statistical software package SPSS-13.0. Initially, the quantitative "RI" study variable was coded (ranging between 0 and 1), and "nodule", as the categorical variable, was classified as malignant or benign. The t-test was applied for determining the presence of statistically significant difference between two independent samples at a significance level of α = 0.05. The normality of both populations was analyzed by the Kolmogorov-Smirnov normality test, while the Levene's test was utilized to evaluate the homocedasticity between both results (benign and malignant lesions). It is important to highlight that both statistical tests correspond to mandatory assumptions for the application of the t-test for comparison of mean values for both populations.
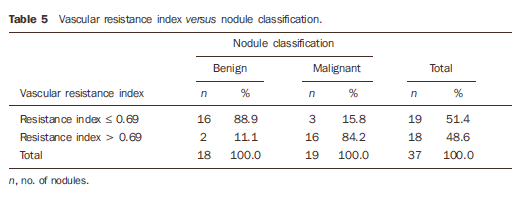
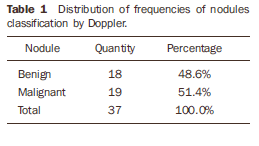
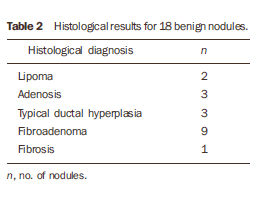
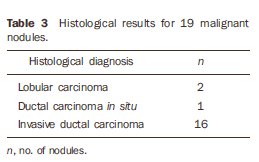
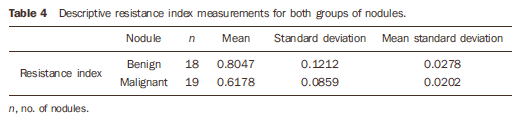
RESULTS Among the 37 biopsied nodules, 19 were diagnosed as malignant, and 18 as benign (Tables 1 to 3).
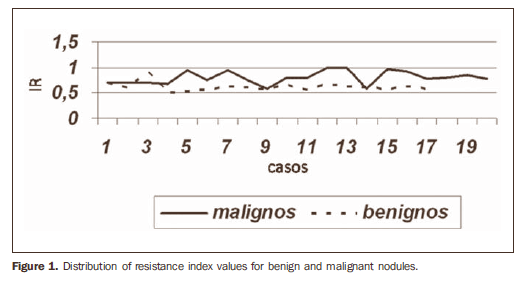
The mean RI was 0.80 for malignant nodules, and 0.61 for benign nodules (Table 4). Figure 1 shows the distribution of the RI values in benign and malignant nodules.
The t-test applied for comparison of two independent samples presented the following result: t = 5.435, with degree of freedom
DISCUSSION Ultrasonography plays a relevant role in the breast imaging evaluation. The technological development can be noticed as a relevant component in the images analysis and processing. In this context, dopplerfluxometry has benefited from the continuous improvement in the temporal resolution of modern ultrasonography equipment. Thus, both the color Doppler signal generated in small vessels and their spectral analysis have demonstrated a significant improvement in the characterization of the blood flow in the breast tissue, allowing a better investigation of the vascularization pattern(16,17). In the present study, the authors evaluated vascular RI of breast nodules greater than 1.0 cm in diameter. Later, the RI data were crossed with the histopathological result for each nodule. A statistically significant difference could be observed for the RI results in relation to the nodules classification (benign or malignant), with the malignant results demonstrating a significantly higher vascular RI as compared with the benign results (0.80 versus 0.61, respectively, with p < 0.001). Similar results have already been observed in some studies utilizing similar methods(18-22). Several studies have analyzed the vascular RI within breast nodules in an attempt to differentiate malignant from benign lesions(18-22). Choi et al. have observed that the RI exceeded 0.70 in more than 80% of patients with malignant nodules with 80.9% sensitivity and 89.1% specificity(20). Peters-Engl et al. have also observed a RI of 0.70 as the best cut-off value to be utilized as an aid in the identification of malignant nodules, with 82% sensitivity, 81% specificity, 70% positive predictive value and 89% negative predictive value(21). In the present study, the method sensitivity for malignant nodules identification was of 84.2%, with 88.9% specificity, 11.1% falsepositive rate, and 15.8% false-negative rate for a RI cut-off value > 0.69, a value practically identical to the ones observed by the above mentioned studies. Almost one decade after the publication of results from relevant studies about the role played by the vascular RI in the evaluation of breast nodules, the authors observed that the present study results were similar to those results, in spite of the significant technological development observed in ultrasonography equipment along this period of time. Consequently, it is understood that such results can be considered as duly validated and that seemingly they are not subjected to the variations resulting from the improvements in both hardware and software directly related to the Doppler function (temporal resolution) in currently available ultrasonography units. Finally, the Doppler technique probably plays a role as an adjuvant to the grayscale in the evaluation of suspicious nodules. It is important to note that this method is not a diagnostic study.
CONCLUSIONS The results of the present study demonstrate that a RI > 0.69 in a nodule > 1 cm suggests a high risk for malignancy, and may represent additional information to be taken into consideration in the selection of lesions eligible for histopathological study .
REFERENCES 1. Souza LRMF, De Nicola H, De Nicola ALA, et al. Nódulos mamários: correlação entre características ultra-sonográficas e achados histológicos em 433 nódulos biopsiados. Rev Imagem. 2005; 27:225-30. [ ] 2. Chala LF, Barros N. Avaliação das mamas com métodos de imagem. Radiol Bras. 2007;40(1):iv-vi. [ ] 3. Roveda Jr D, Piato S, Oliveira VM, et al. Valores preditivos das categorias 3, 4 e 5 do sistema BI-RADS em lesões mamárias nodulares não-palpáveis avaliadas por mamografia, ultra-sonografia e ressonância magnética. Radiol Bras. 2007;40: 93-8. [ ] 4. Fleury EFC, Rinaldi JF, Piato S, et al. Apresentação das lesões mamárias císticas à ultra-sonografia utilizando a elastografia. Radiol Bras. 2008; 41:167-72. [ ] 5. Stavros AT, Thickman D, Rapp CL, et al. Solid breast nodules: use of sonography to distinguish between benign and malignant lesions. Radiology. 1995;196:123-34. [ ] 6. Cosgrove DO, Kedar RP, Bamber JC, et al. Breast diseases: color Doppler US in differential diagnosis. Radiology. 1993;189:99-104. [ ] 7. Raza S, Baum JK. Solid breast lesions: evaluation with power Doppler US. Radiology. 1997; 203:164-8. [ ] 8. Kook SH, Park HW, Lee YR, et al. Evaluation of solid breast lesions with power Doppler sonography. J Clin Ultrasound. 1999;27:231-7. [ ] 9. Mehta TS, Raza S. Power Doppler sonography of breast cancer: does vascularity correlate with node status or lymphatic vascular invasion? AJR Am J Roentgenol. 1999;173:303-7. [ ] 10. Holcombe C, Pugh N, Lyons K, et al. Blood flow in breast cancer and fibroadenoma estimated by colour Doppler ultrasonography. Br J Surg. 1995; 82:787-8. [ ] 11. Yang WT, Metreweli C, Lam PKW, et al. Benign and malignant breast masses and axillary nodes: evaluation with echo-enhanced color power Doppler US. Radiology. 2001;220:795-802. [ ] 12. Birdwell RL, Ikeda DM, Jeffrey SS, et al. Preliminary experience with power Doppler imaging of solid breast masses. AJR Am J Roentgenol. 1997; 169:703-7. [ ] 13. Kubek KA, Chan L, Frazier TG. Color Doppler flow as an indicator of nodal metastasis in solid breast masses. J Ultrasound Med. 1996;15:835-41. [ ] 14. McNicholas MM, Mercer PM, Miller JC, et al. Color Doppler sonography in the evaluation of palpable breast masses. AJR Am J Roentgenol. 1993;161:765-71. [ ] 15. Weinstein SP, Conant EF, Sehgal C. Technical advances in breast ultrasound imaging. Semin Ultrasound CT MR. 2006;27:273-83. [ ] 16. Mehta TS, Raza S, Baum JK. Use of Doppler ultrasound in the evaluation of breast carcinoma. Semin Ultrasound CT MR. 2000;21:297-307. [ ] 17. Tozaki M, Toi M, Miyamoto Y, et al. Power Doppler sonography of breast masses: correlation of Doppler spectral parameters with tumor angiogenesis and histologic growth pattern. J Ultrasound Med. 2000;19:593-600. [ ] 18. Youssefzadeh S, Eibenberger K, Helbich T, et al. Use of resistance index for the diagnosis of breast tumours. Clin Radiol. 1996;51:418-20. [ ] 19. Blohmer JU, Oellinger H, Schmidt C, et al. Comparison of various imaging methods with particular evaluation of color Doppler sonography for planning surgery for breast tumors. Arch Gynecol Obstet. 1999;262:159-71. [ ] 20. Choi HY, Kim HY, Baek SY, et al. Significance of resistive index in color Doppler ultrasonogram: differentiation between benign and malignant breast masses. Clin Imaging. 1999;23:284-8. [ ] 21. Peters-Engl C, Medl M, Leodolter S. The use of colour-coded and spectral Doppler ultrasound in the differentiation of benign and malignant breast lesions. Br J Cancer. 1995;71:137-9. [ ] 22. Chao TC, Lo YF, Chen SC, et al. Color Doppler ultrasound in benign and malignant breast tumors. Breast Cancer Res Treat. 1999;57:193-9. [ ] Received October 13, 2008. * Study developed at Centro Diagnóstico Schmillevitch, São Paulo, SP, Brazil. |
|
Av. Paulista, 37 - 7° andar - Conj. 71 - CEP 01311-902 - São Paulo - SP - Brazil - Phone: (11) 3372-4544 - Fax: (11) 3372-4554
 32 and p-value = 0.000, indicating that, at a significance level corresponding to α = 0.05 the results demonstrated a statistically significant difference pr RI in the malignant nodules as compared with the benign nodules. Considering the variable "nodule type", the cut-off value (RImedian = 0.69) for the classification of benign nodules yielded 88.9% of correct classification (negative for malignancy), and for classification of malignant nodules, 84.2% of correct classification (positive for malignancy), with only 11.1% and 15.8% of false-negative results and false-positive results, respectively (
32 and p-value = 0.000, indicating that, at a significance level corresponding to α = 0.05 the results demonstrated a statistically significant difference pr RI in the malignant nodules as compared with the benign nodules. Considering the variable "nodule type", the cut-off value (RImedian = 0.69) for the classification of benign nodules yielded 88.9% of correct classification (negative for malignancy), and for classification of malignant nodules, 84.2% of correct classification (positive for malignancy), with only 11.1% and 15.8% of false-negative results and false-positive results, respectively (