Radiologia Brasileira - Publicação Científica Oficial do Colégio Brasileiro de Radiologia
AMB - Associação Médica Brasileira CNA - Comissão Nacional de Acreditação
 Vol. 43 nº 2 - Mar. / Apr. of 2010
Vol. 43 nº 2 - Mar. / Apr. of 2010
|
ORIGINAL ARTICLE
|
|
Maximum dilation of the brachial artery in smoking and nonsmoking pregnant and non-pregnant women |
|
|
Autho(rs): Luis Guilherme Carvalho Nicolau, Wellington de Paula Martins, Adilson Cunha Ferreira, Francisco Maximiliano Pancich Gallarreta, Jailson Costa Lima, Daniela de Abreu Barra, Fernando Marum Mauad, Francisco Mauad Filho |
|
|
Keywords: Ultrasonography, Endothelium, Endothelial function, Pregnant women, Smokers |
|
|
Abstract:
IFellow PhD degree, Department of Gynecology and Obstetrics, Faculdade de Medicina de Ribeirão Preto da Universidade de São Paulo (FMRP-USP), Ribeirão Preto, SP, Brazil
INTRODUCTION Smoking is considered the main cause for avoidable deaths in the world and is the second major cause of death, killing one out of ten persons. Currently it is estimated that there are approximately 1.3 billion smokers in the world, with 5 million deaths caused by smoking every year. According to the World Health Organization (WHO), if current trends remain unchanged, close to 10 million people will die every year from 2020 onward(1). In the same manner as smoking, cardiovascular disorder involves high socioeconomic costs and is the first primary cause of death in developed countries. It is known that its physiopathology is basically associated with an alteration of the endothelial function(2) and that the endothelial dysfunction is a systemic process observed in an atherosclerotic environment(3). A normal pregnancy leads to significant changes in the female vascular system and hemodynamics as well as in the endothelium. Simultaneous and subsequent events cause a decrease in the peripheral vascular resistance, as well as a decrease in blood pressure and increase in the cardiac output, with a consequential increase in flow in systemic and pulmonary circulation(4). In the contrary sense, nicotine acts on the maternal circulation by releasing catecholamines, causing an increase in blood pressure, tachycardia, peripheral vasoconstriction and a decrease in the placental flow, resulting in decreased oxygenation and compromising fetal nutrition(5). Additionally, nicotine causes autonomic imbalance, irregularities in the coronary blood flow and endothelial dysfunction(6,7). The consequences of smoking on pregnancy and on the cardiovascular dynamics are the motivating factors for the study of the influence of tobacco on the endothelium. A noninvasive method for the evaluation of the endothelial function by means of ultrasonography, proposed by Celermajer et al.(6), is recommended by the International Brachial Artery Reactivity Task Force and is expressed as a change in the brachial artery diameter as a percentage of its basal diameter(8). The international terminology used for the method is FMD(6,8,9), an acronym for flow-mediated dilation. However, some disagreement is observed among Brazilian studies, with two terms being utilized: FMD(10,11), as "flow-mediated dilation", and another term simply called DILA(12–15). In the present study, the FMD acronym is adopted. The objective of the present study is to evaluate the alterations in the endothelial function induced by cigarette smoking (maximum time of brachial artery dilation) in pregnant and non-pregnant women.
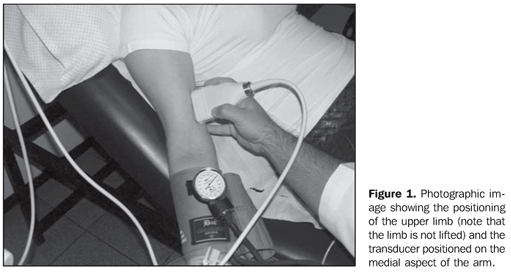
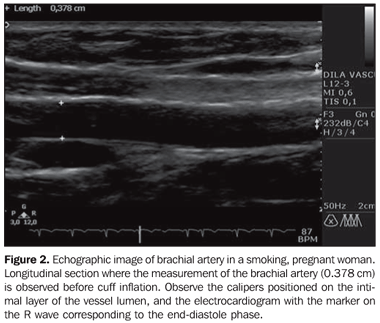
MATERIALS AND METHODS Subjects Pregnant women were invited to participate in the study, with gestational ages ranging between 24 and 28 weeks, and non-pregnant women, all of them with ages ranging between 20 and 30 years, who were voluntarily submitted obstetric and gynecologic ultrasonography in the period from July 2007 to February 2009. A total sample of 133 women was selected and divided into four groups as follows: pregnant women (n = 80) [nonsmokers (n = 47) and smokers (n = 33)] and non-pregnant women (n = 53) [nonsmokers(n = 34) and smokers (n = 19)]. Smoking participants, both pregnant and non-pregnant women were questioned on the approximate number of smoked cigarettes per day. All the participants signed a term of free and informed consent, and the study was approved by the Committee for Ethic in Research of the Institution. Sonographic study For the FMD evaluation, all the patients were asked to have their last pre-study meal no later than 10:00 PM on the previous day, as well as avoiding ingestion of alcohol and alcohol-containing products, or other products containing caffeine or chocolate. The smoking pregnant and non-pregnant women were asked to refrain from smoking after 10:00 PM of the previous day. Before being positioned for the sonographic study, all the patients were measured and weighted for calculation of their body mass index, besides having the blood pressure measured. Once they were positioned, the patients were monitored with the electrocardiograph coupled with the HD 11 ultrasonography apparatus (Philips Medical System; Bothel, WA, USA), remaining in rest for 10 minutes or more in a room at a temperature of 22 ± 2°C and dimmed lighting, in order to provide good relaxation conditions. The next step was the placement of the linear transducer on the medial aspect of the arm, at 5 to 10 cm above the antecubital fold, insonating the brachial artery posteriorly to the biceps and laterally to the brachial muscle (Figure 1). A standardized measurement limited by intima/lumen interfaces was adopted as parameter for the measurement of the brachial artery diameter. The measurement was made taking as a reference the end-diastolic phase represented by the R wave at the electrocardiogram. Before the cuff inflation, a 5-second film was acquired for later measurement of basal brachial artery diameter (Figure 2).
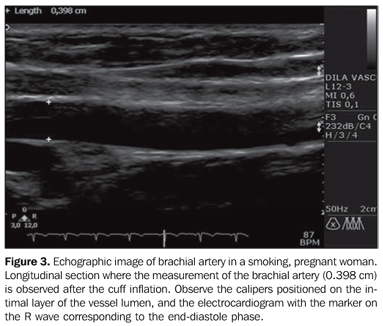
The pressure of the sphygmomanometer cuff inflation on the right forearm was standardized at 200 mmHg for all the patients, with the occlusion being maintained for five minutes. After cuff deflation, four 5-second films were acquired at 30, 60, 90 and 120 seconds, for later measurement of post-occlusion arterial diameter. For all five acquired films, three measurements were performed on the R wave, with a sharper brachial artery image (Figure 3). All the sonographic images were recorded in the apparatus itself and later the data were transferred to a Microsoft Excel worksheet (Microsoft Corporation; Redmond, WA, USA).
The mean value for the three measurements of the arterial diameter was considered as the final value. For the FMD calculation, the following formula was utilized for each one of the time spans (30, 60, 90 and 120 seconds): FMD (%) = [(final diameter of the brachial artery) – (initial diameter of the brachial artery)] / (initial diameter of the brachial artery × 100). Statistics The non-paired t-test was utilized in the comparison among groups, as the variables presented a parametric distribution. For the evaluation of maximum brachial artery dilation at different time spans (30, 60, 90 and 120 seconds) the ANOVA repeated measures test was utilized. In all these tests, a significance level of 5% was adopted. The softwares SPSS 16.0 for Windows (SPSS Inc.; Chicago, IL, USA) and GraphPad 5.0 for Windows (GraphPad Software; San Diego, CA, USA) were utilized for data analysis.
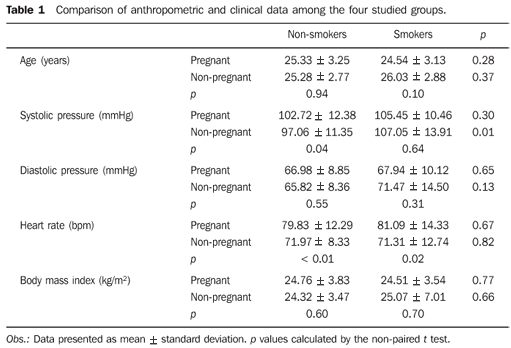
RESULTS As regards age, no statistically significant difference was observed in any of the comparisons (Table 1). As regards gestational age, the groups were similar, with no significant difference between nonsmoking (25.19 ± 1.13) and the smoking pregnant women (25.44 ± 1.29). Among the smokers, the pregnant women smoked more cigarettes per week (110.51 ± 58.14) as compared with the non-pregnant ones (78.52 ± 45.17), with a statistically significant difference (p = 0.04).
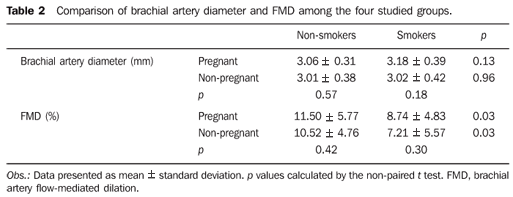
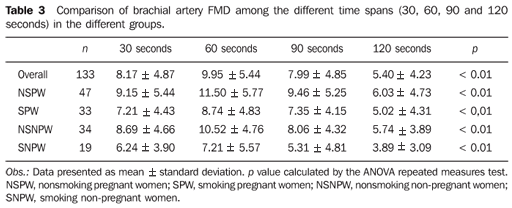
As regards to systolic arterial pressure (SAP, in mmHg), no significant difference was observed between smoking and nonsmoking pregnant women. The smoking non-pregnant women presented higher SAP than the nonsmoking non-pregnant ones (p = 0.01). Also, a statistically significant difference was observed when comparing nonsmoking pregnant and non-pregnant women: the pregnant ones presented higher SAP than the non-pregnant ones (p = 0.04) (Table 1). The use of cigarettes had no significant effect on SAP among pregnant and non-pregnant women. No significant difference was observed in relation to diastolic arterial pressure (DAP) and body mass index among pregnant and non-pregnant participants, smokers and non-smokers as described on Table 1. The heart rate was higher among the smoking and in nonsmoking pregnant women, but no significant difference was observed between these two groups (Table 1). As regards the basal brachial artery diameter, no significant difference was observed in any of the comparisons (Table 2). In the evaluation of the time at which maximum brachial artery dilation was achieved, this was observed at 60 seconds after the cuff deflation when the whole sample was analyzed in all the groups (Figure 3 and Table 3).
After the maximum dilation was established, the FMD was compared among the groups, being greater among the nonsmoking pregnant women as compared with smoking pregnant participants. The result was similar for the non-pregnant group: the nonsmoking participants had greater FMD than the smoking participants (p = 0.03) (Table 2).
DISCUSSION As the technique was applied with cuff inflation on the forearm, the whole sample demonstrated maximum and significant dilation at 60 seconds. Studies have demonstrated that the maximum brachial artery dilation occurs around 60 seconds after cuff deflation(8,16–18); other studies based on such evidences only use this time as a reference to obtain FMD(19–21). These two factors motivated the evaluation of cigarette smoking influence on the maximum dilation, and if such time should be the reference when one studies the FMD and the smoking habit. For the nonsmoking group, it was expected that the maximum dilation occurred at 60 seconds or close to this time spam, which would be in agreement with studies that demonstrate maximum dilation between 45 and 75 seconds(8,16). Among nonsmoking pregnant women, studies demonstrate that maximum dilation is also close to this time(22,23). As regards cigarette influence, a study on brachial artery dilation in 11-year-old children exposed to cigarette smoke, has demonstrated that maximum dilation is delayed in the group with highest exposure as compared with the less exposed one, in which the maximum dilation is observed at around 60 seconds(17). After the determination of maximum dilation, the FMD difference among groups was evaluated, observing that both smoking and nonsmoking pregnant women presented smaller FMD as compared with control groups. Studies have demonstrated that FMD decreases in active smokers(24,25), as well as in passive smokers(26). As regards smoking pregnant women, a study has also demonstrated a decrease in FMD in this group(9). Considering that the main objective of the present study was the evaluation of cigarette smoking on the endothelium, a well defined age group was selected (20 to 30 years) so that aging would not affect the FMD(27). Likewise, all the pregnant women were submitted to evaluation between the 24th and 28th gestational weeks, to avoid the influence of gestational age on the FMD, considering that studies demonstrate differences in FMD at the different gestational ages(28,29). No difference was observed in SAP among smoking and nonsmoking pregnant women, which did not occur among the non-pregnant women. As the DAP is analyzed, no significant difference was found in any group. This can be explained by the fact that the patients went through an abstinence period, as it was expected that there would be a difference in arterial pressure between smoking and nonsmoking pregnant women(30,31). The fact of having developed the study with normotensive pregnant women was important to avoid interference in FMD, as hypertension may play an influence on it(32). The results of the present study demonstrated higher heart rate among the pregnant women, both in the smoking and nonsmoking groups. It was actually expected that in the smoking group the heart rate would be higher(30,31), however no significant difference was observed. This can be explained by the fact that the participants were oriented to refrain from smoking for a period, a fact that has been demonstrated by other authors(9,33). As the body mass index was analyzed, no interference on FMD was expected, as all the patients in the sample presented a body mass index < 30 kg/m2(34). As regards the brachial artery diameter, no significant difference among groups was observed; therefore it was expected that there would be no interference on FMD, as studies had demonstrated that the higher is the basal brachial artery diameter, the lower is FMD(20,24). This is a technique that requires training, besides an accurate technical skill to be performed. The investigator must be skilled, and appropriate equipment coupled with electrocardiograph is required. The assessment of brachial artery flow-mediated dilation is another useful tool to detect individuals with cardiovascular diseases, and with the standardization of the technique, FMD tends to become a widely accepted technique for evaluating the endothelial function.
CONCLUSION Maximum brachial artery dilation was achieved at 60 seconds, independently of being the women pregnant or not. However, the FMD was smaller among the smoking women, pregnant or not, demonstrating that smoking compromises the endothelial function.
REFERENCES 1. World Health Organization (WHO). TFI, 2009. [acessado em 27 de agosto de 2009]. Disponível em: http://www.who.int/tobacco/global_data/country_profiles/Introduction.pdf [ ] 2. Fenster BE, Tsao PS, Rockson SG. Endothelial dysfunction: clinical strategies for treating oxidant stress. Am Heart J. 2003;146:218–26. [ ] 3. Korkmaz H, Onalan O. Evaluation of endothelial dysfunction: flow-mediated dilation. Endothelium. 2008;15:157–63. [ ] 4. Williams JK, Anthony MS, Honoré EK, et al. Regression of atherosclerosis in female monkeys. Arterioscler Thromb Vasc Biol. 1995;15:827–36. [ ] 5. Nakamura MU, Alexandre SM, Kuhn dos Santos JF, et al. Obstetric and perinatal effects of active and/or passive smoking during pregnancy. São Paulo Med J. 2004;122:94–8. [ ] 6. Celermajer DS, Sorensen KE, Gooch VM, et al. Non-invasive detection of endothelial dysfunction in children and adults at risk of atherosclerosis. Lancet. 1992;340:1111–5. [ ] 7. Adamopoulos D, van de Borne P, Argacha JF. New insights into the sympathetic, endothelial and coronary effects of nicotine. Clin Exp Pharmacol Physiol. 2008;35:458–63. [ ] 8. Corretti MC, Anderson TJ, Benjamin EJ, et al. Guidelines for the ultrasound assessment of endothelial-dependent flow-mediated vasodilation of the brachial artery: a report of the International Brachial Artery Reactivity Task Force. J Am Coll Cardiol. 2002;39:257–65. [ ] 9. Quinton AE, Cook CM, Peek MJ. The relationship between cigarette smoking, endothelial function and intrauterine growth restriction in human pregnancy. BJOG. 2008;115:780–4. [ ] 10. Fernandes JBF, Soares GM, Martins WP, et al. Obesidade e alteração da estrutura arterial em mulheres jovens com síndrome dos ovários micropolicísticos. Rev Bras Ginecol Obstet. 2009 31:342–8. [ ] 11. Martins WP, Soares GM, Vieira CS, et al. Resistência à insulina em mulheres com síndrome dos ovários policísticos modifica fatores de risco cardiovascular. Rev Bras Ginecol Obstet. 2009;31:111–6. [ ] 12. Castro PT, Montenegro CAB, Carvalho ACP, et al. Dilatação fluxo-mediada da artéria braquial em mulheres com artrite reumatóide. Radiol Bras. 2007;40:247–50. [ ] 13. Regattieri NAT. Dilatação fluxo-mediada da artéria braquial. Desenvolvimento da técnica, estudo em pacientes de risco para aterosclerose e em um grupo controle [resumo de tese]. Radiol Bras. 2005;38:388. [ ] 14. Meirelles CM, Leite SP, Montenegro CAB, et al. Confiabilidade da medida da dilatação fluxo-mediada da artéria braquial pela ultra-sonografia. Arq Bras Cardiol. 2007;89:176–83. [ ] 15. Garrido KU, Rezende Filho J, Leite SP, et al. Dilatação fluxo-mediada da artéria braquial: estudo da função endotelial em mulheres na menopausa. Rev Bras Ecocardiogr. 2008;21:22–6. [ ] 16. Hashimoto M, Miyamoto Y, Matsuda Y, et al. New methods to evaluate endothelial function: non-invasive method of evaluating endothelial function in humans. J Pharmacol Sci. 2003;93:405–8. [ ] 17. Kallio K, Jokinen E, Raitakari OT, et al. Tobacco smoke exposure is associated with attenuated endothelial function in 11-year-old healthy children. Circulation. 2007;115:3205–12. [ ] 18. Uehata A, Lieberman EH, Gerhard MD, et al. Noninvasive assessment of endothelium-dependent flow-mediated dilation of the brachial artery. Vasc Med. 1997;2:87–92. [ ] 19. Martins WP, Nastri CO, Vieira CS, et al. Flow-mediated dilatation in polycystic ovary syndrome women. Fertil Steril. 2009;91:e23; author reply e24. [ ] 20. Martins WP, Nastri CO, Ferriani RA, et al. Brachial artery pulsatility index change 1 minute after 5-minute forearm compression: comparison with flow-mediated dilatation. J Ultrasound Med. 2008;27:693–9. [ ] 21. Soares GM, Vieira CS, Martins WP, et al. Increased arterial stiffness in nonobese women with polycystic ovary syndrome (PCOS) without comorbidities: one more characteristic inherent to the syndrome? Clin Endocrinol (Oxf). 2009;71:406–11. [ ] 22. Savvidou MD, Kametas NA, Donald AE, et al. Non-invasive assessment of endothelial function in normal pregnancy. Ultrasound Obstet Gynecol. 2000;15:502–7. [ ] 23. Quinton AE, Cook CM, Peek MJ. A longitudinal study using ultrasound to assess flow-mediated dilatation in normal human pregnancy. Hypertens Pregnancy. 2007;26:273–81. [ ] 24. Celermajer DS, Sorensen KE, Georgakopoulos D, et al. Cigarette smoking is associated with dose-related and potentially reversible impairment of endothelium-dependent dilation in healthy young adults. Circulation. 1993;88(5 Pt 1):2149–55. [ ] 25. Celermajer DS, Sorensen KE, Bull C, et al. Endothelium-dependent dilation in the systemic arteries of asymptomatic subjects relates to coronary risk factors and their interaction. J Am Coll Cardiol. 1994;24:1468–74. [ ] 26. Celermajer DS, Adams MR, Clarkson P, et al. Passive smoking and impaired endothelium-dependent arterial dilatation in healthy young adults. N Engl J Med. 1996;334:150–4. [ ] 27. Juonala M, Kähönen M, Laitinen T, et al. Effect of age and sex on carotid intima-media thickness, elasticity and brachial endothelial function in healthy adults: the cardiovascular risk in Young Finns Study. Eur Heart J. 2008;29:1198–206. [ ] 28. Valtonen P, Laitinen T, Lyyra-Laitinen T, et al. Serum L-homoarginine concentration is elevated during normal pregnancy and is related to flow-mediated vasodilatation. Circ J. 2008;72:1879–84. [ ] 29. Saarelainen H, Laitinen T, Raitakari OT, et al. Pregnancy-related hyperlipidemia and endothelial function in healthy women. Circ J. 2006;70:768–72. [ ] 30. Benowitz NL. The role of nicotine in smoking-related cardiovascular disease. Prev Med. 1997;26:412–7. [ ] 31. Rosemberg J. Nicotina – droga universal. [acessado em 27 de agosto de 2009]. Disponível em: http://www.inca.gov.br/tabagismo/publicacoes/nicotina.pdf [ ] 32. Moriguchi J, Itoh H, Harada S, et al. Low frequency regular exercise improves flow-mediated dilatation of subjects with mild hypertension. Hypertens Res. 2005;28:315–21. [ ] 33. Mauad Filho F, Sá AE, Gross R, et al. Efeito do tabagismo sobre a freqüência cardíaca materna e fetal. Rev Bras Ginecol Obstet. 1983;5:177–81. [ ] 34. Chung S, Yoon IY, Shin YK, et al. Endothelial dysfunction and inflammatory reactions of elderly and middle-aged men with obstructive sleep apnea syndrome. Sleep Breath. 2009;13:11–7. [ ] Received October 19, 2009. * Study developed at Escola de Ultrassonografia e Reciclagem Médica Ribeirão Preto (EURP), Ribeirão Preto, SP, Brazil. Financial support: Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (Capes). |
|
Av. Paulista, 37 - 7° andar - Conj. 71 - CEP 01311-902 - São Paulo - SP - Brazil - Phone: (11) 3372-4544 - Fax: (11) 3372-4554