Radiologia Brasileira - Publicação Científica Oficial do Colégio Brasileiro de Radiologia
AMB - Associação Médica Brasileira CNA - Comissão Nacional de Acreditação
 Vol. 41 nº 2 - Mar. / Apr. of 2008
Vol. 41 nº 2 - Mar. / Apr. of 2008
|
REVIEW ARTICLE
|
|
Liver neoplasms: imaging characterization |
|
|
Autho(rs): Dario Ariel Tiferes, Giuseppe D'Ippolito |
|
|
Keywords: Liver, Neoplasm, Magnetic resonance imaging, Ultrasound, Computed tomography |
|
|
Abstract:
IMD, Radiologist at Fleury Medicina e Saúde, São Paulo, SP, Brazil
INTRODUCTION A wide range of benign and malignant tumors occurs in the liver. Although the characterization of focal hepatic lesions may represent a challenge for radiologists, most of lesions can be diagnosed on the basis of imaging findings. The present study is aimed at reviewing the most frequent imaging findings related to benign and malignant hepatic tumors in adult individuals. This paper describes the main characteristics of hemangioma, focal nodular hyperplasia, adenoma, hepatocellular carcinoma, intrahepatic cholangiocarcinoma and hepatic metastasis observed at ultrasonography (US) computed tomography (CT) and magnetic resonance imaging (MRI).
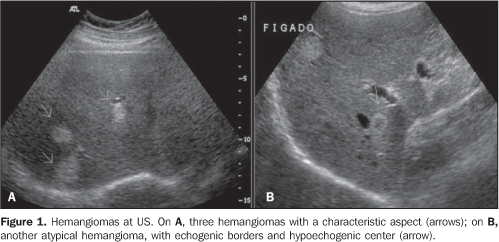
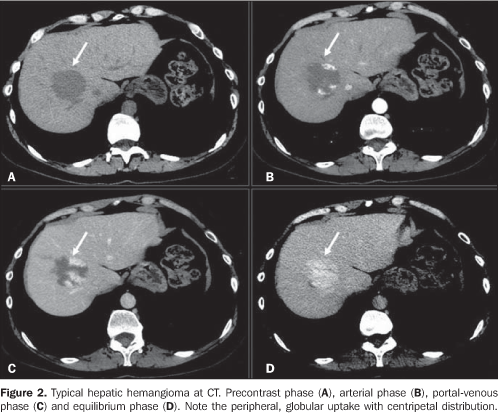
HEMANGIOMA Hemangioma is the most frequent benign tumor of the liver, with an incidence ranging between 0.4% and 20% in autopsy studies(1,2), and representing a very frequent incidental finding on imaging studies, particularly US. In most cases, hemangiomas are small (< 3.0 cm), and may be multiple in up to 50% of patients(3). Microscopically, hemangiomas consist of vascular spaces irregular in size, lined by a single layer of endothelial cells and separated by fibrous septa(4). The finding of a hyperechogenic, homogeneous and well–defined nodule at US is highly indicative of hemangioma(3) (Figure 1). TC findings include the presence of hypodense lesion in the precontrast phase of the study. After intravenous injection of iodinated contrast agent, the typical enhancement pattern includes peripheral globular enhancement in the arterial and portal phases, progressive centripetal enhancement and persistence of enhancement in the equilibrium phase(3,5,6) (Figure 2). A complete non–opacification of the lesion does not hinder the diagnosis of hemangioma. Frequently, large lesions may not be completely enhanced and the center of the lesion remains hypodense, possibly representing fibrosis, former hemorrhage and cystic alterations(7,8).
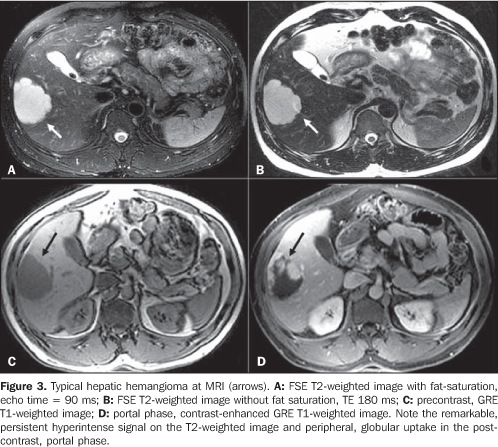
Small hemangiomas (< 1.5 cm) frequently are early and completely contrast–enhanced. This enhancement pattern results from the small vascular spaces size causing an increase in the inside blood (and contrast mean) flow velocity. The persistence of the contrast–enhancement during the equilibrium phase is useful in the differentiation of hemangiomas from other hypervascular lesions such as focal nodular hyperplasia and some types of metastases presenting a more rapid wash–out pattern(7). At MRI, hemangiomas are characterized by lesions with well–defined margins, hyperintense signal on T2–weighted sequences persisting with high echo times (around 180 ms)(2,5). The gadolinium uptake pattern is similar to the iodinated contrast–mean uptake pattern found at CT (Figure 3). With T2–weighted sequences and intravenous, paramagnetic contrast–enhanced dynamic study, MRI sensitivity and specificity in the diagnosis of hemangiomas reach 98%(5).
Despite its high specificity in the diagnosis of hemangiomas, radionuclide scintigraphy(9) is not routinely utilized because of its low accuracy in the diagnosis of small and/or multiple lesions. Hemangiomas may present atypical, although quite suggestive sonographic patterns. For example, they may present an echogenic halo and a hypoechogenic center(10) (Figure 1). Giant hemangiomas (typically with > 6 cm or 10 cm) frequently present heterogeneous echotexture, with a hypoechoic center. Additionally, in steatotic livers, hemangiomas frequently present as a hypoechogenic nodule as a result of the high hepatic parenchymal echogenicity. Most hemangiomas with an atypical pattern at US present typical intravenous contrast uptake patterns at CT and MRI(7,8). Other less frequent findings of hemangiomas include calcified, hyalinized and cystic/multilocular lesions. Hemangiomas also may be exophytic or pedunculated, and not infrequently present small, adjacent arterial–portal venous shunt determining transitory perfusional disorder in the adjacent hepatic parenchyma observed at CT and MRI(7).
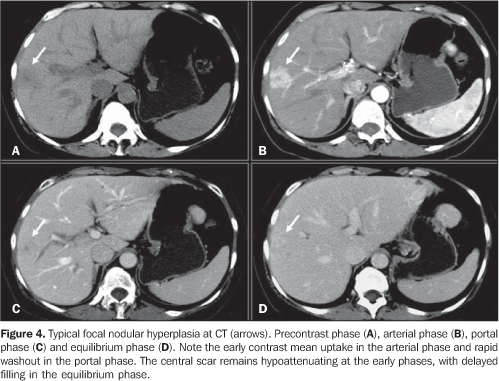
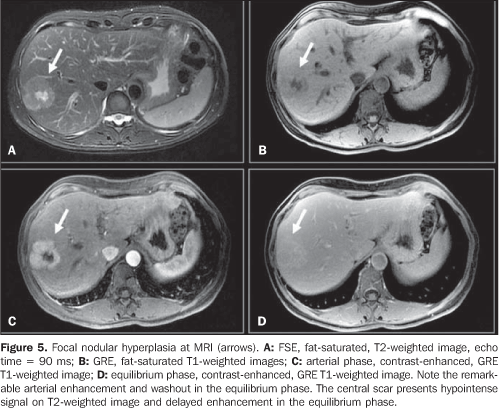
FOCAL NODULAR HYPERPLASIA Focal nodular hyperplasia (FNH) is defined as a nodule constituted by apparently normal hepatocytes, occurring in a liver with a normal histological aspect. It is the second more frequent benign hepatic tumor, with a reported incidence of 0.9%, predominantly in women (female–to–male ratio = 8:1) and in young patients. About 20% of patients present with multiple lesions and in association with hepatic hemangiomas(11). Histologically, FNH may be classified into classic (80%) and non–classic (20%) presentations. The classic form presents three components: abnormal nodular architecture, vascular malformations and proliferation of biliary ducts. The non–classic form presents two of the three components, including the ductal proliferation(11). FNH pathogenesis is still to be completely understood. Vascular malformation and/or vascular injury are suggested as probable mechanisms for its development(12). Association with steroids is controversial. This tumor is usually asymptomatic and, in this case, it does not require any treatment(13). Usually, FNH is an incidental finding at imaging studies, especially after the dissemination and improvement of intravenous contrast–enhanced dynamic CT and MRI studies(14,15). At US, this lesion presents a non–specific pattern, being poorly visualized. Generally, this lesion presents like a slightly hypoechogenic or hyperechogenic nodule and cannot be definitively characterized by this method(15). Generally, classic FNH can be effectively characterized at CT and MRI. Currently, helical CT and particularly multidetector CT allow a multiphase hepatic study (arterial, portal and equilibrium phases of hepatic contrast–enhancement), that is indispensable in the evaluation and correct characterization of the tumor vascularization. Typical CT findings of FNH include lobulated, well–defined lesion, isoattenuating or slightly hypoattenuating in the precontrast phase, and a significant, homogeneous contrast–enhancement in the arterial phase, with rapid washout in the portal and equilibrium phases. Usually, a small central, star–like scar consisting of vascular malformations is observed, tending to delayed contrast–enhancement(14,15) (Figure 4).
At MRI, classic FNH is depicted as a slightly hypointense lesion on T–weighted images, and with a subtle hyperintensity on T2–weighted images. In 85% of these lesions, a central scar can be identified with a higher intensity signal than the rest of the lesion on T2–weighted images. The pattern of intravenous contrast–enhancement of FNH is similar to the one described for CT images(14). In the presence of such characteristics, the MRI diagnostic specificity reaches 98%(14) (Figure 5).
Atypical FNHs may present as large, heterogeneous lesions in multiple sites. The tumor may present a lower degree of contrast–enhancement, absence of the central scar enhancement and delayed contrast–enhancement of the pseudocapsules. Exceptionally, central punctiform calcifications may be observed(15). Differentiating FNH from other benign (adenomas) and malignant lesions (hepatocarcinomas, fibrolamellar carcinomas and hypervascularized metastases) may be extremely difficult, requiring histopathological study.
ADENOMA Hepatocellular adenoma is a rare benign neoplasm, usually found in women with a history of long–term use of oral contraceptives(16). Although the pathogenic mechanism of this lesion is still to be completely understood, the utilization of estrogenic or androgenic substances, particularly over long periods of time, increases significantly the incidence of this tumor(16). Patients with glycogen storage disease also present an increased risk for developing adenomas(17). Adenomas have been most frequently detected as incidental findings in patients submitted to multi–phase, contrast–enhanced CT or MRI studies for other unrelated signs or symptoms. Approximately 70% of adenomas are solitary and 30%, multiple(18,19). The patients use to be asymptomatic, with normal results for laboratory hepatic function tests and alpha–fetoprotein levels. Very large adenomas may present intratumoral bleeding and rupture, causing abdominal pain and hypotension(20). Histologically, adenomas consist of large cords of cells resembling normal hepatocytes separated by dilated sinusoids with arterial perfusion. Portal venous supply and biliary ducts are absent(21). Adenomas cells contain large amounts of glycogen and lipid, the latter rarely macroscopically found(19). Most rarely, this lesion may undergo malignant degeneration to hepatocellular carcinoma, despite former long–term stability. Significant increase in the size of the tumor and in alpha–fetoprotein serum levels suggests a diagnosis of malignant transformation(22,23). The term hepatic adenomatosis is applied to cases of multiple adenomas (> 10) found in patients without known risk factors, and possibly representing a distinctive entity(24). In spite of presenting histologic characteristics similar to solitary adenomas, hepatic adenomatosis may present a high potential for growth, hemorrhage and, eventually malignant transformation(21). Adenomas can be detected at US, but normally do not present a typical echographic pattern, and requires supplementary CT or MRI for a better evaluation of the lesion(23). Imaging findings indicating the diagnosis of adenoma at multi–phase, contrast–enhanced CT include the presence of a solitary (or, eventually, multiple) lesion with well–defined borders, and sometimes a pseudocapsule. The presence of fat or intralesional hemorrhage foci is quite characteristic. The lesion tends to be isoattenuating to the hepatic parenchyma in the precontrast phase, with homogeneous contrast–enhancement in the arterial phase, tending to become isoattenuating to the hepatic parenchyma in the portal and equilibrium phases(23). At MRI, imaging findings suggesting adenoma include hyperintense signal on T1–weighted images and slightly hyperintense on T2–weighted images. The signal loss on out–of–phase, gradient–echo sequences indicates the presence of intralesional fat, and constitutes a sign suggesting the diagnosis of adenoma. The pattern of paramagnetic contrast–enhancement is similar to the one observed at CT(18,25) (Figure 6). Very heterogeneous adenomas presenting atypical findings may require additional studies (MRI utilizing hepato–specific contrast agents), or even biopsy to rule–out the possibility of malignancy. Differential diagnoses for adenomas include other hypervascular lesions that occur in young adults without association with other hepatopathies, such as FNH, fibrolamellar hepatocarcinoma and metastasis(23). Prognosis in cases of adenomas is still to be fully established. In many cases, adenomas may remain stable for long periods of time, or even may regress with the discontinuation of the estrogen medication. Episodes of hemorrhage and malignant transformation, although rare, remain as the main clinical problems. Some criteria indicating surgical resection of adenomas include large lesions (> 5 cm in diameter) and presence of symptoms related to intratumoral hemorrhage(20,23). In patients with a high number of lesions (adenomatosis), liver transplant has been proposed, considering its higher risk for malignancy(18,20).
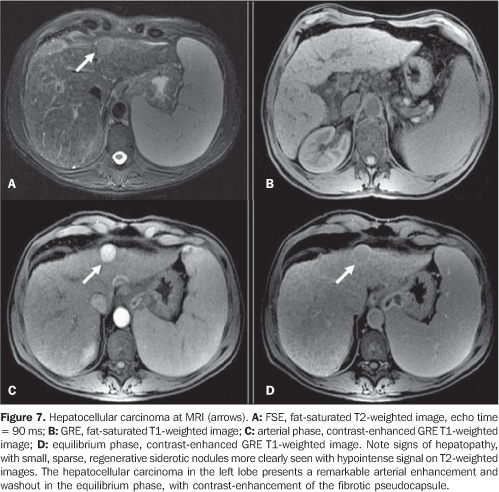
HEPATOCELLULAR CARCINOMA Hepatocellular carcinoma (HCC) generally occurs as a complication from hepatic cirrhosis, particularly the one caused by hepatitis B and C viruses(26,27), with a prevalence in cirrhotic livers removed at transplantation reaching 14%(28). Patients with hepatic cirrhosis can be evaluated by US, CT and MRI. Although each of these methods presents a particularity, the capacity of detecting a focal lesion depends on the contrast between the lesion and the parenchyma, which may be affected by the presence of fat, necrosis and fibrosis. Hepatic alterations related to fibrosis and nodular regeneration associated to perfusional alterations resulting from portal hypertension, usually present in these patients, represent a challenge to radiologists in the detection and characterization of HCC at the different imaging methods. Ultrasound, particularly, presents significant limitations in the evaluation of cirrhotic nodules in the liver. Considering the higher risk of cirrhotic patients for developing HCC, upon detection of any solid nodule at US, the investigation should proceed with CT or MRI. It is essential that both CT and MRI are performed utilizing intravenous contrast agents, and including multi–phase studies in the contrast–enhanced arterial phase for detection and characterization of HCC(27). Nodular lesions in a cirrhotic liver may be classified into two major categories: regenerative nodules and dysplastic or neoplastic nodules(29). Regenerative nodules represent parenchymal areas increased as a response to necrosis and circulatory alterations. Nodules > 3–5 mm are called macroregenerative nodules, but rarely reach more than 20 mm. Generally, nodules with > 20 mm are dysplastic. Regenerative nodules may contain iron and, in this case, they are called siderotic nodules(29). Although regenerative nodules are present in all of cirrhotic livers, they are seen in a minority of patients at CT, and in about 50% o cases at MRI, the siderotic nodules being more clearly seen(27,30). Siderotic nodules may present spontaneously hyperattenuating at non–contrast–enhanced CT, and, normally, at MRI present with hypointense signal on T2–weighted sequences, as a function of the presence of iron inside them. On T1–weighted sequences, such nodules use to present a subtle hypointense or isointense signal to the surrounding hepatic parenchyma and, less frequently, hyperintense signal. Typically, these nodules do not enhance at contrast–enhanced CT in the arterial phase, and in the portal–venous phase they enhance homogeneously, similarly to the hepatic parenchyma; thus they are indistinguishable(27) (Figure 7).
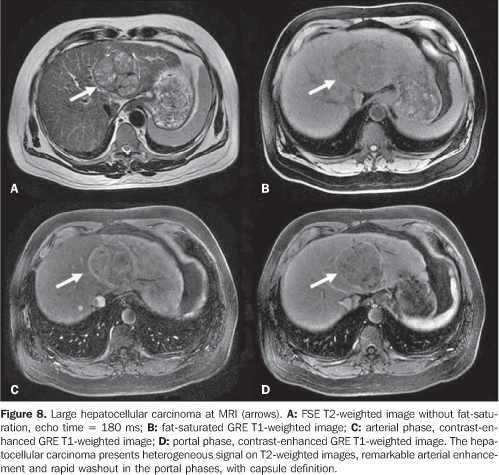
Dysplastic nodules present morphological characteristics occupying an intermediate position among the characteristics found in regenerative nodules and HCC. One of the classifications adopted includes low–grade dysplastic nodules, high–grade dysplastic nodules, high–grade dysplastic nodules with HCC foci, and the HCC itself. Histologic studies demonstrate that through this progression, the number of portal tracts decrease and new arterial vessels develop within the lesions. These are determining findings in the characterization of these lesions(27,29). Despite the utilization of multi–phase, contrast–enhanced techniques including acquisition in the hepatic arterial phase, CT and MRI still lack accuracy in the detection of HCC. Correlation studies evaluating livers removed from patients submitted to transplant have demonstrated CT and MRI sensitivity in the detection of HCC of 59%–68% and 50%, respectively. Additionally, the sensitivity in the detection of the total number of lesions was 37%–44% for CT, and 50% for MRI(28,31). These results reflect the inclusion of many nodules < 1.0 cm in diameter, remarkably difficult to be detected by imaging methods. HCC presents a variable appearance at CT and MRI. Although the hyperintense signal on MRI T2–weighted sequences suggests HCC, the sinal may present as hypo, iso–, or hyperintense in relation to the adjacent liver on T1– and T2–weighted images(27,31,32). Most of times, small HCCs are hypervascularized, with preferential enhancement in the arterial phase, and wash–out in the portal–venous phase imaging, where the attenuation/signal is similar to the one of the liver (Figure 7). A minority of tumors is hypovascular, and is more clearly identified in the portal–venous and equilibrium phases imaging(28,31). Large lesions (> 5.0 cm) tend to be heterogeneous, and may present necrosis, fatty metamorphosis and tumor capsule(27) (Figure 8). In principle, any vascular nodule found in a patient with hepatopathy should be considered as highly suspect for HCC, particularly those with > 2.0 cm in diameter. A sign observed at contrast–enhanced CT and MRI may help in the diagnosis of other hepatic lesions (for example, regenerative or dysplastic nodules). With a certain frequency, HCCs present a fibrotic pseudocapsule that is enhanced in delayed phases or may present low–contrast–enhancement in the equilibrium phase, in comparison with the adjacent hepatic parenchyma(32).
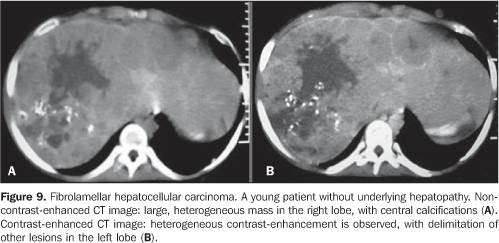
Some authors advocate the utilization of arteriography with lipiodol, a substance that preferentially links to tumor cells and can be seen at delayed CT studies (at least three weeks after intra–arterial lipiodol injection)(33). However, this method has not been frequently utilized in the diagnosis of HCC, considering that, besides its invasiveness, the intense uptake by the lesions impairs the management of the progression and viability of the lesion. So lipiodol has been utilized in association with chemotherapic drugs and hepatic artery embolization, as palliative therapeutic measures for non–resectable hepatic tumors or in patients waiting for liver transplantation. Despite difficult, the evaluation of the treatment efficacy, must be performed by means of the study of intravenous contrast–enhanced areas, which usually translates into tumor viability(33). Screening for HCC is indicated for patients undergoing clinical conditions allowing curative treatment, taking age, presence of comorbidities and degree of the hepatic function involvement into consideration. Although screening for HCC with US and alpha–fetoprotein dosage every 6 months is usual in our environment, randomized studies are still required to evaluate the efficacy and cost–benefit ratio of these studies. In some regions of the world where the prevalence of HCC is high, screening tests are more rigorous, including alpha–fetoprotein dosage every two months, abdominal ultrasound every three months, and CT or MRI every six months(26). Fibrolamellar hepatocarcinoma is an uncommon type of hepatocarcinoma with clinical, prognostic and histopathologic features different from classic HCC found in cirrhotic livers. This lesion occurs preferentially in young patients with no history of underlying hepatopathies and absence of serum tumor markers(34). Frequent imaging findings of fibrolamellar HCC include large, well–defined, lobulated, heterogeneous masses in non–cirrhotic livers, with radiate septa and a central scar with fibrotic components. Calcifications (better evaluated by CT) are seen in about 50% of cases, almost exclusively in the central scar regions. Intravenous contrast–enhanced dynamic studies demonstrate preferentially arterial, heterogeneous vascularization. On delayed images, there is a trend to persistent contrast uptake by the central scar, demonstrating the fibrotic component of this region (Figure 9). At MRI, the tumor generally presents hypointense signal on T1–weighted sequences, and hyperintense signal on T2–weighted sequences. The central scar presents hypointense signal on T2–weighted sequences. Such finding is useful in the differentiation of other tumors that may present with central scars, especially in cases of FNH where the central scar presents hyperintense signal on MRI T2–weighted sequences(34).
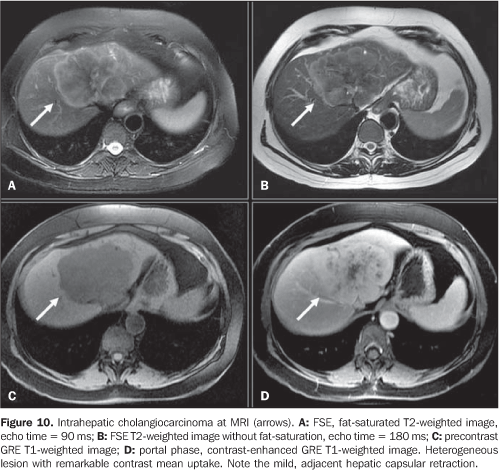
CHOLANGIOCARCINOMA Although biliary tract tumors are not included in the scope of the present study, imaging findings of some intrahepatic cholangiocarcinomas are described, considering that they should be included in the differential diagnosis of primary hepatic lesions. Cholangiocarcinoma is an adenocarcinoma originating in the epithelium of the biliary ducts. It is the second primary malignant tumor most frequently found in the liver, after HCC. It is associated to intrahepatic lithiasis, choledocal cyst, Caroli's disease, primary sclerosing cholangitis, and C. sinensis infection(35). The tumor may originate in any portion of the biliary epithelium, and may be classified into intrahepatic (peripheral or hilar) or extrahepatic. Peripheral cholangiocarcinoma originates in secondary intrahepatic ducts and hilar cholangiocarcinoma originates in the right and left hepatic ducts or their confluence, receiving the name of Klatskin tumor(36). Peripheral cholangiocarcinomas usually present as a solid, well–defined, lobulated mass with peripheral contrast–uptake at CT and MRI. Generally, it appears as a bulky tumor at the moment of the diagnosis, because of the absence of symptoms in the early phases of the disease, where frequently dilatation of the biliary tract is not found, in contrast to the hilar presentation. Focal dilatation of biliary ducts and capsular retraction usually is found in 30% of cases (Figure 10). The persistent contrast–uptake by the tumor in delayed phases is a finding frequently described as a result of the presence of intralesional fibrotic tissue(37–40).
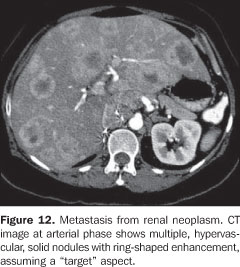
HEPATIC METASTASES Hepatic metastasis is the most frequent found malignant lesion of the liver. A correct diagnosis is essential for determining the therapeutic conduct and prognosis. Accurate information on the number of lesions, as well as their extent constitute a prerequisite for the success of the surgical resection and therapeutic monitoring. Besides the screening for hepatic metastasis, the oncologic patient requires the differentiation between hepatic metastasis and other benign hepatic nodules frequently, incidentally found on imaging studies(41). Ultrasound presents a limited sensitivity for detecting hepatic metastasis, ranging between 50% and 70%(42). Most of times, small metastases, especially those < 1.0 cm or isoechoic to the hepatic parenchyma, are not detectable by US. The most typical US finding in cases of hepatic metastasis is a hypo or isoechoic lesion surrounded by a hyperechoic halo giving the lesion the so called "target" or bull's eye" aspect. The presence of a halo has a high sensitivity for the diagnosis of malignancy (about 85%)(43). Despite the lower cost and higher availability, this method presents a lower reproducibility as compared with CT and MRI, difficulting the management of the lesion progression(42,43). CT in association with MRI is considered as the main imaging method for screening hepatic metastases in oncologic patients, considering the better spatial resolution and higher sensitivity (about 75%) and specificity in the detection and characterization of focal hepatic lesions. Additionally, CT allows the evaluation of further associated diffuse, hepatic alterations, besides studying the whole abdomen. The helical technology, especially with multi–detector tomographs, allows the performance of multi–phase (hepatic precontrast, arterial, portal–venous and equilibrium phases) contrast–enhanced and non–contrast–enhanced hepatic studies, which is essential for an appropriate evaluation of the liver, particularly in the context of malignancy. Additionally an appropriate evaluation of the hepatic portal, arterial and venous threes can be performed. 3D angiographic reconstruction can be performed, allowing the correlation between the lesions and the major vascular branches(44,45). Most metastases are hypovascular and present as hypoattenuating nodules in relation to the hepatic parenchyma in the portal–venous phase, with heterogeneous or ring–shaped contrast–enhancement (Figure 11). Some neoplasms (for example: renal cell carcinomas, thyroid carcinomas, breast carcinomas, carcinoid tumors, neuroendocrine tumors and melanomas) may develop hypervascular hepatic metastases which can be more clearly identified in the arterial phase because of the early and transitory contrast agent uptake, tending to become isoattenuating to the parenchyma in the portal–venous phase(44,45) (Figure 12).
Small hypoattenuating nodules, particularly those with < 1.0 cm, may be hardly characterized at CT. In these cases, MRI can aid in the diagnostic evaluation, considering the method specificity in the characterization of small cysts and hemangiomas usually present, including in the group of oncologic patients(46). MRI presents sensitivity (about 75%) and specificity very similar to CT in the evaluation of secondary hepatic lesions. Similarly to CT, the conventional technique for evaluation of the liver utilizes intravenous paramagnetic contrast agent, with series acquired during the hepatic arterial, portal–venous and equilibrium phases. T2–weighted images are extremely important in the characterization of lesions, and represent an additional advantage as compared with CT, particularly in the case of small lesions(42,46) (Figure 11). The utilization of hepato–specific contrast agents, such as superparamagnetic iron oxide, seems to increase the accuracy of this method in the detection of metastases, especially the small ones(47). However, taking its high cost into consideration, this type of contrast agent has not been routinely utilized.
CONCLUSION With the dissemination of imaging diagnosis devices as well as their increasingly widespread utilization, the occurrence of incidentally found hepatic nodules has become more and more frequent in patients submitted to imaging studies or screening for tumors(48–51). The technological development of these apparatuses has allowed the detection of increasingly smaller lesions, difficulting their characterization that later might be affected by the findings of pseudo–hepatic lesions at contrast–enhanced CT and MRI(52,53). Recognizing the main imaging findings of the most frequent hepatic tumors and some of their characteristics(54) may positive and definitely help the radiologist in the approach and management of this group of patients.
REFERENCES 1. Edmondson HA, Peters RL. Neoplasms of the liver. In: Schift L, Schift ET, editors. Diseases of the liver. Philadelphia: Lippincott; 1982. p. 1114–45. [ ] 2. Tiferes DA, D'Ippolito G, Szejnfeld J. Ressonância magnética dos hemangiomas hepáticos: avaliação das características morfológicas e quantitativas. Radiol Bras. 2003;36:1–9. [ ] 3. Nelson RC, Chezmar JL. Diagnostic approach to hepatic hemangiomas. Radiology. 1990;176:11–3. [ ] 4. Kojimahara M. Ultrastructural study of hemangiomas. 4. Cavernous hemangioma of the liver. Acta Pathol Jpn. 1986;36:1477–85. [ ] 5. Semelka RC, Brown ED, Ascher SM, et al. Hepatic hemangiomas: a multi–institutional study of appearance on T2–weighted and serial gadolinium–enhanced gradient–echo MR images. Radiology. 1994;192:401–6. [ ] 6. Leslie DF, Johnson CD, MacCarty RL, et al. Single–pass CT of hepatic tumors: value of globular enhancement in distinguishing hemangiomas from hypervascular metastases. AJR Am J Roentgenol. 1995;165:1403–6. [ ] 7. Vilgrain V, Boulos L, Vullierme MP, et al. Imaging of atypical hemangiomas of the liver with pathologic correlation. Radiographics. 2000;20: 379–97. [ ] 8. D'Ippolito G, Appezzato LF, Ribeiro ACR, et al. Apresentações incomuns do hemangioma hepático: ensaio iconográfico. Radiol Bras. 2006;39: 219–25. [ ] 9. Birnbaum BA, Weinreb JC, Megibow AJ, et al. Definitive diagnosis of hepatic hemangiomas: MR imaging versus Tc–99m–labelled red blood cell SPECT. Radiology. 1990;176:95–101. [ ] 10. Moody AR, Wilson SR. Atypical hepatic hemangioma: a suggestive sonographic morphology. Radiology. 1993;188:413–7. [ ] 11. Nguyen BN, Fléjou JF, Terris B, et al. Focal nodular hyperplasia of the liver: a comprehensive pathologic study of 305 lesions and recognition of new histologic forms. Am J Surg Pathol. 1999; 23:1441–54. [ ] 12. Wanless IR, Mawdsley C, Adams R. On the pathogenesis of focal nodular hyperplasia of the liver. Hepatology. 1985;5:1194–200. [ ] 13. Mathieu D, Kobeiter H, Maison P, et al. Oral contraceptive use and focal nodular hyperplasia of the liver. Gastroenterology. 2000;118:560–4. [ ] 14. Mortelé KJ, Praet M, Van Vlierberghe H, et al. CT and MR imaging findings in focal nodular hyperplasia of the liver: radiologic–pathologic correlation. AJR Am J Roentgenol. 2000;175:687–92. [ ] 15. Hussain SM, Terkivatan T, Zondervan PE, et al. Focal nodular hyperplasia: findings at state–of–the–art MR imaging, US, CT, and pathologic analysis Radiographics. 2004;24:3–17. [ ] 16. Soe KL, Soe M, Gluud C. Liver pathology associated with the use of anabolic–androgenic steroids. Liver. 1992;12:73–9. [ ] 17. Labrune P, Trioche P, Duvaltier I, et al. Hepatocellular adenomas in glycogen storage disease type I and III: a series of 43 patients and review of the literature. J Pediatr Gastroenterol Nutr. 1997;24:276–9. [ ] 18. Paulson EK, McClellan JS, Washington K, et al. Hepatic adenoma: MR characteristics and correlation with pathologic findings. AJR Am J Roentgenol. 1994;163:113–6. [ ] 19. Ichikawa T, Federle MP, Grazioli L, et al. Hepatocellular adenoma: multiphasic CT and histopathologic findings in 25 patients. Radiology. 2000; 214:861–8. [ ] 20. Ault GT, Wren SM, Ralls PW, et al. Selective management of hepatic adenomas. Am Surg. 1996;62:825–9. [ ] 21. Grazioli L, Federle MP, Ichikawa T, et al. Liver adenomatosis: clinical, histopathologic, and imaging findings in 15 patients. Radiology. 2000; 216:395–402. [ ] 22. Foster JH, Berman MM. The malignant transformation of liver cell adenomas. Arch Surg. 1994; 129:712–7. [ ] 23. Grazioli L, Federle MP, Brancatelli G, et al. Hepatic adenomas: imaging and pathologic findings. Radiographics. 2001;21:877–92. [ ] 24. Fléjou JF, Barge J, Menu Y, et al. Liver adenomatosis: an entity distinct from liver adenoma? Gastroenterology. 1985;89:1132–8. [ ] 25. Arrivé L, Fléjou JF, Vilgrain V, et al. Hepatic adenoma: MR findings in 51 pathologically proved lesions. Radiology. 1994;193:507–12. [ ] 26. El–Serag HB, Mason AC. Rising incidence of hepatocellular carcinoma in the United States. N Engl J Med. 1999;340:745–50. [ ] 27. Baron RL, Peterson MS. From the RSNA refresher courses: Screening the cirrhotic liver for hepatocellular carcinoma with CT and MR imaging: opportunities and pitfalls. Radiographics. 2001;21:117–32. [ ] 28. Peterson MS, Baron RL, Marsh JW Jr, et al. Pretransplantation surveillance for possible hepatocellular carcinoma in patients with cirrhosis: epidemiology and CT–based tumor detection rate in 430 cases with surgical pathologic correlation. Radiology. 2000;217:743–9. [ ] 29. International Working Party. Terminology of nodular hepatocellular lesions. Hepatology. 1995; 22:983–93. [ ] 30. Murakami T, Nakamura H, Hori S, et al. CT and MRI of siderotic regenerating nodules in hepatic cirrhosis. J Comput Assist Tomogr. 1992;16:578–82. [ ] 31. Krinsky GA, Lee VS, Theise ND, et al. Hepatocellular carcinoma and dysplastic nodules in patients with cirrhosis: prospective diagnosis with MR imaging and explantation correlation. Radiology. 2001;219:445–54. [ ] 32. D'Ippolito G, Abreu L, Borri ML, et al. Apresentações incomuns do hepatocarcinoma: ensaio iconográfico. Radiol Bras. 2006;39:137–43. [ ] 33. Cammà C, Schepis F, Orlando A, et al. Transarterial chemoembolization for unresectable hepatocellular carcinoma: meta–analysis of randomized controlled trials. Radiology. 2002;224:47–54. [ ] 34. Ichikawa T, Federle MP, Grazioli L, et al. Fibrolamellar hepatocellular carcinoma: imaging and pathologic findings in 31 recent cases. Radiology. 1999;213:352–61. [ ] 35. Sherlock S, Dooley J. Disease of the liver and biliary system. 10th ed. London: Blackwell; 1997. [ ] 36. Nakanuma Y, Minato H, Kida T, et al. Pathology of cholangiocellular carcinoma. In: Tobe T, Kameda H, Okudaira M, et al., editors. Primary liver cancer in Japan. Tokyo: Springer–Verlag; 1994. p. 39–50. [ ] 37. Kim TK, Choi BI, Han JK, et al. Peripheral cholangiocarcinoma of the liver: two–phase spiral CT findings. Radiology. 1997;204:539–43. [ ] 38. Lee JW, Han JK, Kim TK, et al. CT features of intraductal intrahepatic cholangiocarcinoma. AJR Am J Roentgenol. 2000;175:721–5. [ ] 39. Choi BI, Han JK, Kim TK. Benign and malignant tumors of the biliary tree. In: Gazelle SG, editor. Hepatobiliary and pancreatic radiology. New York: Thieme; 1998. p. 630–76. [ ] 40. Choi BI, Han JK, Kim TK. Diagnosis and staging of cholangiocarcinoma by computed tomography. In: Meyers MA, editor. Neoplasms of the digestive tract: imaging, staging and management. Philadelphia: Lippincott–Raven; 1998. p. 503–16. [ ] 41. Sahani DV, Kalva SP. Imaging the liver. Oncologist. 2004;9:385–97. [ ] 42. Kinkel K, Lu Y, Both M, et al. Detection of hepatic metastases from cancers of the gastrointestinal tract by using noninvasive imaging methods (US, CT, MR imaging, PET): a meta–analysis. Radiology. 2002;224:748–56. [ ] 43. Wernecke K, Vassallo P, Bick U, et al. The distinction between benign and malignant liver tumors on sonography: value of a hypoechoic halo. AJR Am J Roentgenol. 1992;159:1005–9. [ ] 44. Bree RL, Greene FL, Ralls PW, et al. Suspected liver metastases. American College of Radiology. ACR Appropriateness Criteria. Radiology. 2000; 215:213–24. [ ] 45. Soyer P, Poccard M, Boudiaf M, et al. Detection of hypovascular hepatic metastases at triple–phase helical CT: sensitivity of phases and comparison with surgical and histopathologic findings. Radiology. 2004;231:413–20. [ ] 46. Mueller GC, Hussain HK, Carlos RC, et al. Effectiveness of MR imaging in characterizing small hepatic lesions: routine versus expert interpretation. AJR Am J Roentgenol. 2003;180:673–80. [ ] 47. Hagspiel KD, Neidl KF, Eichenberger AC, et al. Detection of liver metastases: comparison of superparamagnetic iron oxide–enhanced and unenhanced MR imaging at 1.5 T with dynamic CT, intraoperative US, and percutaneous US. Radiology. 1995;196:471–8. [ ] 48. Machado MM, Rosa ACF, Lemes MS, et al. Hemangiomas hepáticos: aspectos ultra–sonográficos e clínicos. Radiol Bras. 2006;39:441–6. [ ] 49. Machado MM, Rosa ACF, Barros N, et al. Múltiplos pequenos nódulos hepáticos hiperecogênicos sem reverberação sonora posterior: outra forma de apresentação dos hamartomas dos ductos biliares. Radiol Bras. 2005;38:389–91. [ ] 50. Machado MM, Rosa ACF, Herman P, et al. Avaliação dos tumores hepáticos ao Doppler. Radiol Bras. 2004;37:371–6. [ ] 51. Machado MM, Rosa ACF, Barros N, et al. Hemangiomas hipoecogênicos. Radiol Bras. 2003;36: 273–6. [ ] 52. Meirelles GSP, Tiferes DA, D'Ippolito G. Pseudolesões hepáticas na ressonância magnética: ensaio iconográfico. Radiol Bras. 2003;36:305–9. [ ] 53. Meirelles GSP, D'Ippolito G. Pseudolesões hepáticas na tomografia computadorizada helicoidal: ensaio iconográfico. Radiol Bras. 2003;36:229–35. [ ] 54. Bezerra ASA, D'Ippolito G, Martelli P, et al. Calcificações hepáticas: freqüência e significado. Radiol Bras. 2003;36:199–205. [ ] Received June 8, 2007. Accepted after revision August 28, 2007. * Study developed in the Department of Imaging Diagnosis at Escola Paulista de Medicina/Universidade Federal de São Paulo (Unifesp/EPM) and Fleury Medicina e Saúde, São Paulo, SP, Brazil. |
|
Av. Paulista, 37 - 7° andar - Conj. 71 - CEP 01311-902 - São Paulo - SP - Brazil - Phone: (11) 3372-4544 - Fax: (11) 3372-4554