Radiologia Brasileira - Publicação Científica Oficial do Colégio Brasileiro de Radiologia
AMB - Associação Médica Brasileira CNA - Comissão Nacional de Acreditação
 Vol. 41 nº 2 - Mar. / Apr. of 2008
Vol. 41 nº 2 - Mar. / Apr. of 2008
|
ORIGINAL ARTICLE
|
|
Survey on quality control of radiopharmaceutical dose calibrators in nuclear medicine units in the city of São Paulo, SP, Brazil |
|
|
Autho(rs): Ana Carolina Moreira de Bessa, Alessandro Martins da Costa, Linda V. E. Caldas |
|
|
Keywords: Nuclear medicine, Dose calibrators, Calibration, Quality control |
|
|
Abstract:
IMaster degree in Nuclear Technology, Fellow PhD degree, Instituto de Pesquisas Energéticas e Nucleares – Comissão Nacional de Energia Nuclear (IPEN–CNEN), São Paulo, SP, Brazil
INTRODUCTION A dose calibrator is an essential device in a nuclear medicine unit, utilized for determining the activity of radiopharmaceuticals (pharmaceuticals labeled with radionuclides) administered to patients both for diagnostic and therapeutic purposes. Essentially, it consists of a well–type ionization chamber coupled with a special electronic circuit which allows the display of the instrument response in activity units. Should the instrument indicate an activity value lower than the actual value, the patient will be given a radiopharmaceutical with a higher–than–prescribed activity; being unnecessarily exposed to extra radiation. On the other hand, should the instrument indicate an activity value higher than the actual value, the administered activity will be insufficient, requiring repetition of the procedure and also implying an unnecessary increase in dose to the patient and to occupationally exposed individuals involved in the process. Aiming at assuring a satisfactory performance of the dose calibrator utilized in a nuclear medicine unit, the Comissão Nacional de Energia Nuclear (CNEN) — National Commission of Nuclear Energy — determines that these instruments are semestrally submitted to accuracy, precision and linearity tests, and daily to reproducibility tests(1). Although CNEN does not require geometry testing, it is recommended that this test is performed at dose calibrators installation(2–5). On activity measurements, accuracy describes the degree of agreement between a measurement result and an actual activity value; precision describes the degree of agreement between results of successive activity measurements performed under identical conditions, and repeated at short time intervals. Reproducibility tests are related to long term dose calibrators stability. If a same measurement can be reproduced over a period of several half–lives of a radioactive source, the instrument response will be rated as linear, indicating, for a particular radionuclide, the range where the radionuclide activity can be correctly estimates (linearity). Dose calibrators manufacturers usually utilize radionuclide solutions in a determined container to define the original calibration of the device. Other containers utilized in nuclear medicine units, such as plastic or glass syringes may present different volumes and absorption properties, so other correction factors should be determined for such containers, consisting in geometry testing of the instruments. Usually, commercial dose calibrators are originally calibrated by manufacturers with standard radionuclide solutions (direct calibration) of a national or traceable standards laboratory, or, alternatively, by comparison with a reference instrument (indirect calibration). In this type of calibration (indirect), a comparison between the reading of the instrument to be calibrated and the reading of the directly calibrated reference instrument is made by introducing a reference source under identical conditions for measurement in each ionization chamber well. Operational conditions of the source to be measured are applied, and the reading of the first instrument is adjusted, or a correction factor is obtained. The calibration or recalibration of a dose calibrator may be based on the accuracy tests with clinically significant radionuclide sources. Currently, the standard CNEN–NN–3.05(1) requires only accuracy tests with reference 57Co, 133Ba or 137Cs sources, and not with clinically significant radionuclide sources. Those three sources cover the range of utilization of a dose calibrator, and are assigned to simulate the geometry of a short–half–life radionuclide solution in a similar container. The 57Co source simulates a 99mTc source, the 133Ba source simulates a 131I source, and the 137Cs source simulates a 99Mo source. Should a dose calibrator accuracy is tested with a 57Co source, for example, the appropriate operational condition is selected for 57Co and not for 99mTc. As a result, the instrument is not tested for the portion of its electronic circuit dedicated to the measurement of 99mTc activity. Generally, this shortcoming is observed for all of the clinically significant radionuclide sources. Therefore, significant errors may arise from activity measurements utilizing operational conditions of dose calibrators which are not directly tested for accuracy. This instruments calibration or recalibration also may be undertaken by means of intercomparison. Studies published in the period between 2003 and 2005(6–10) demonstrate the increasing necessity of implementing a program of quality control for dose calibrators in nuclear medicine units. So, a survey on quality control tests and their respective frequency was undertaken in nuclear medicine units in the city of São Paulo. A preliminary survey on the accuracy of activity measurements in dose calibrators also was undertaken with seven devices, utilizing clinically significant radionuclide sources in the Laboratório de Calibração de Instrumentos (LCI) (Calibration Laboratory) of Instituto de Pesquisas Energéticas e Nucleares (IPEN).
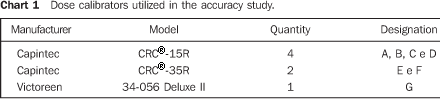
MATERIALS AND METHODS Initially, a survey on dose calibrators existing in the city of São Paulo was undertaken. This survey was based on a questionnaire sent with a form to 87 nuclear medicine units. Later, a second questionnaire with questions regarding dose calibrators quality control were sent to those unities which had answered the first questionnaire. A survey on the accuracy of activity measurements performed with dose calibrators was undertaken with seven instruments called A, B, C, D, E, F and G, in the LCI–IPEN. These dose calibrators are described on Chart 1. These dose calibrators accuracy was tested for 67Ga, 99mTc and 201Tl sources. These clinically utilized radionuclide sources were provided by the IPEN Centro de Radiofarmácia in cylindrical, flat–bottomed, colorless glass flasks with 47.0 mm in height, 23.5 mm external diameter, and wall thickness of 1.1 mm. These are the IPEN standard flasks for radiopharmaceuticals supply. The samples volume was 4 ml.
The accuracy of dose calibrators was indirectly checked, utilizing a reference instrument. The readings of the instrument under test and of the reference instrument were compared by means of a same source under identical measurement conditions in each ionization chamber well. The percentage deviation between the average of activities measured on the instrument under test and the average of activities measured on the reference instrument was utilized for determining the accuracy of the tested instrument. The accuracy test acceptance limit recommended by the standard CNEN–NN–3.05(1) is ± 10%. The NPL–CRC dose calibrator (Southern Scientific Ltd.), of the LCI–IPEN, was utilized as reference system. This dose calibrator was calibrated by the primary standard laboratory in England — National Physical Laboratory — and therefore it is a secondary standard system.
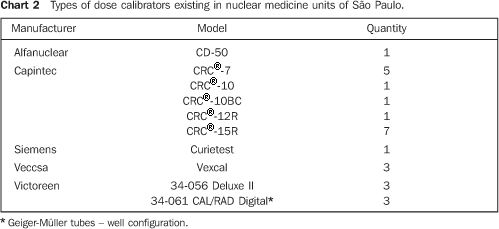
RESULTS Among 87 nuclear medicine units included in the survey on dose calibrators in the city of São Paulo, only 22 filled in and returned the forms about 26 calibrators. No type of response or communication was received from the other 65 units contacted. The results are shown on Chart 2.
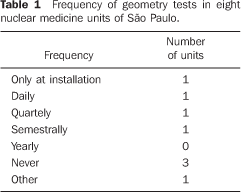
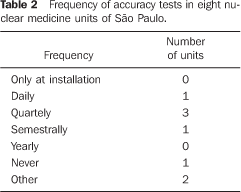
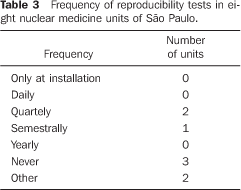
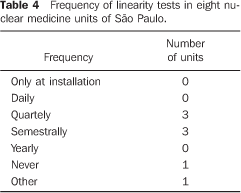
Among the 22 nuclear medicine units which had returned the firs form/questionnaire, only eight filled in and returned the second form/questionnaire with questions about dose calibrators quality control. No type of response or communication was received from the other 14 units. Among the eight nuclear medicine units which had returned the form, three reported the unavailability of reference standard sources for quality control tests; one of them utilizing reference standard sources borrowed from other nuclear medicine units to perform quality control tests, one performed only a semestral linearity test, and one did not perform any test. The frequency of quality control tests in these nuclear medicine units is shown on Tables 1 to 4.
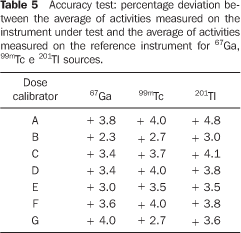
The results of the survey on accuracy of activity measurements performed with seven dose calibrators in the LCI–IPEN, utilizing 67Ga, 99mTc and 201Tl as reference sources are shown on Table 5. In all of the measurements, the percentage deviation between the average of activities measured on the instrument under test and the average of activities measured on the reference instrument was < 5%.
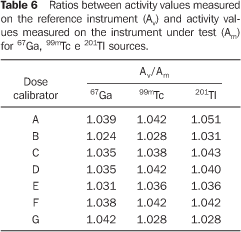
As already mentioned, the calibration of a dose calibrator may be based on an accuracy test. Table 6 presents the ratios between the values of the measured activities of the reference instrument and those of the instrument under test. Uncertainties were not assigned to these values, considering that type B uncertainties(11) in measurements with the reference dose calibrator were not estimated. These values determine correction factors, that is to say, numeric factors by which the noncorrected results are to be multiplied to balance systematic errors, establishing a calibration (or recalibration) of the dose calibrators tested for the radionuclides utilized.
DISCUSSION There are 26 dose calibrators in the 22 nuclear medicine units which filled in and returned the form/questionnaire on dose calibrators existing in the city of São Paulo (Chart 2). About 58% of these dose calibrators are manufactured by Capintec. Table 1 demonstrates that only one of the eight nuclear medicine units which filled in and returned the second forms/questionnaires with questions related to dose calibrators quality control, had performed the geometry test at the device installation, and three had never performed such test; in only one of the units the accuracy test is semestrally performed, and in four this test is more frequently performed (Table 2). Among the tests required by the standards CNEN–NN–3.05(1), the reproducibility test is the less frequently performed, as shown on Table 3. Reproducibility tests are particularly significant for evaluating the equipment operation constancy over the time for different measurements conditions. Linearity test is semestrally performed in three nuclear medicine units, and quarterly in three (Table 4). Tables 1 to 4 results show some inappropriatenesses, for example, daily geometry tests in one unit (Table 1) and lack of daily reproducibility tests in all of the nuclear medicine units (Table 3). Maybe this is due to the lack of technical and bibliographic support for users of dose calibrators in nuclear medicine units in Brazil. The lack of technical support is a result from the distance between manufacturers and users, considering that this type of instrument is not manufactured in Brazil. On the other hand, the lack of bibliographic support could be solved with the cooperation of specialists from several institutions of the country, developing a publication including detailed information on quality control tests, defining the most appropriate format for data recording and presentation as well as tests frequency, following the example of other countries like Spain, with the study developed by Aguado et al.(4), and England, with the study developed by Gadd et al.(5). Results included in Table 5 demonstrate that all of the tested dose calibrators meet the acceptance criteria (± 10%) defined by the standard CNEN–NN–3.05 for accuracy tests. Mean values of the ratios between the activities measured on the reference instrument and activites measured on the instrument under test, calculated as per Table 6 are 1.035 ± 0.06, 1.037 ± 0.06 and 1.039 ± 0.08, respectively for 67Ga, 99mTc e 201Tl sources. Based on these values, one may be observe that there is trend to underestimate the values of activities measured for 67Ga, 99mTc e 201Tl sources. This trend has also been observed in other studies developed in Brazil(7).
CONCLUSIONS Approximately 75% of nuclear medicine units in the city of São Paulo have not answered the first questionnaire about the types of existing equipment. Sixty–four percent of the nuclear medicine units which had answered the first questionnaire, did not answered the second one with questions related to dose calibrators quality control. Considering that no step was taken to minimize the lack of responses, the conclusions of the present study were limited by the size of the sampling. Notwithstanding, it may be concluded that the current situation of dose calibrators quality control in Brazil is unsatisfactory. Accuracy tests were performed with seven dose calibrators for radionuclide sources with clinical purposes. This accuracy survey has not indicated any performance faults, and has established the calibration of these instruments for the utilized sources. Dose calibrators must be calibrated with clinically utilized radionuclide sources, although this is not required by the standard CNEN–NN–3.05(1). This procedure can be undertaken by the LCI–IPEN.
Acknowledgements The authors thank Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) and Fundação de Amparo à Pesquisa do Estado de São Paulo (Fapesp) for the partial financial support.
REFERENCES 1. Comissão Nacional de Energia Nuclear. CNEN–NN–3.05: Requisitos de radioproteção e segurança para serviços de medicina nuclear. Resolução CNEN–10/96. Diário Oficial da União, 19/4/1996. [ ] 2. Dansereau RN, Methe BM. Dose variance associated with calibration and administration of radiopharmaceuticals. Am J Health Syst Pharm. 2001;58:580–4. [ ] 3. Costa AM, Caldas LVE. Intercomparação e calibração de medidores de atividade utilizados em serviços de medicina nuclear. Radiol Bras. 2003; 36:293–7. [ ] 4. Aguado MM, García AD, Navarro AR, et al. Control de calidad de activímetros. Rev Esp Med Nucl. 2004;23:434–43. [ ] 5. Gadd R, Baker M, Nijran KS, et al. Measurement good practice guide no. 93: protocol for establishing and maintaining the calibration of medical radionuclide calibrators and their quality control. Teddington: National Physical Laboratory; 2006. [ ] 6. Joseph L, Anuradha R, Nathuram R, et al. National intercomparisons of 131I radioactivity measurements in nuclear medicine centres in India. Appl Radiat Isot. 2003;59:359–62. [ ] 7. Santos JA, Iwahara A, Oliveira AE, et al. National intercomparison program for radiopharmaceutical activity measurements. Appl Radiat Isot. 2004;60:523–7. [ ] 8. Kim GY, Lee HK, Jeong HK, et al. Comparison of radioactivity measurements with radionuclide calibrators in the Republic of Korea. Appl Radiat Isot. 2005;63:201–5. [ ] 9. Oropesa P, Hernández AT, Serra R, et al. Comparisons of activity measurements with radionuclide calibrators – a tool for quality assessment and improvement in nuclear medicine. Appl Radiat Isot. 2005;63:493–503. [ ] 10. van Wyngaardt WM, Simpson BRS. Preparation and use of standards for a comparison exercise among users of 131I capsules in South Africa. Phys Medica. 2005;21:101–5. [ ] 11. Associação Brasileira de Normas Técnicas. Instituto Nacional de Metrologia, Normatização e Qualidade Industrial. Guia para a expressão da incerteza de medição. 3ª ed. Rio de Janeiro: ABNT, Inmetro; 2003. [ ] Received April 17, 2007. Accepted after revision September 10, 2007. * Study developed at Instituto de Pesquisas Energéticas e Nucleares – Comissão Nacional de Energia Nuclear (IPEN–CNEN), São Paulo, SP, Brazil. |
|
Av. Paulista, 37 - 7° andar - Conj. 71 - CEP 01311-902 - São Paulo - SP - Brazil - Phone: (11) 3372-4544 - Fax: (11) 3372-4554