Radiologia Brasileira - Publicação Científica Oficial do Colégio Brasileiro de Radiologia
AMB - Associação Médica Brasileira CNA - Comissão Nacional de Acreditação
 Vol. 41 nº 2 - Mar. / Apr. of 2008
Vol. 41 nº 2 - Mar. / Apr. of 2008
|
ORIGINAL ARTICLE
|
|
Study of nuchal translucency, ductus venosus, nasal bone and maternal age for detection of fetal chromosomal disorders in a high-risk population |
|
|
Autho(rs): Antonio Carlos Vieira Lopes, Kleber Pimentel, Maria Betânia Pereira Toralles, Alessandro de Moura Almeida, Luciana Vieira Lopes, Edward Araújo Júnior, Luciano Marcondes Machado Nardozza, Antonio Fernandes Moron |
|
|
Keywords: Nuchal translucency measurement, Ductus venosus, Nasal bone, Maternal age, Trisomy |
|
|
Abstract:
IPhD, Associate Professor, Head for Department of Gynecology, Obstetrics & Human Reproduction at Faculdade de Medicina da Universidade Federal da Bahia (UFBA), Salvador, BA, Brazil
INTRODUCTION In 1866, Langdon Down observed that, in individuals with trisomy 21, the skin seemed to be redundant for the body, the nose was small and the face was flat(1). During the last years, several studies have proposed non–invasive techniques for the prenatal diagnosis of chromosomal abnormalities based on both sonographic findings and maternal biochemical tests. Randomized studies have demonstrated that invasive studies for karyotype research, such as amniocentesis and chorionic villus sampling, present a risk for miscarriage around 1%(2,3). In Brazil, maternal biochemical tests are restricted to few private and high–cost health centers, so ultrasonography remains as the main method for screening chromosomal abnormalities. Advanced maternal age (MA) represents a risk factor for chromosomal abnormalities (trisomies 13, 18 and 21). Considering the high incidence of intrauterine death of fetuses with chromosomal abnormalities, the risk decreases with the progression of the gestational age(4,5). Nuchal translucency (NT) consists of a hypoechoic sonographic image resulting from accumulation of fluid behind the neck of a fetus, occurring more exuberantly between the 10th and 14th weeks of gestation(6). Since early nineties, several studies have reported the association between nuchal translucency thickness and the presence of chromosomal abnormalities(7,8). Early fetal cardiac failure has been proposed as a possible mechanism for increase in the NT thickness(9). Therefore, the evaluation of the ductus venosus (DV) flow pattern may reflect this hemodynamic status. Studies in the literature report that alterations in the DV flow pattern (absent or reverse flow) present a high sensitivity with low rate of false–positive results in the detection of chromosomal abnormalities(10). Also, the presence of an abnormal ductus venosus flow pattern in a fetus with increased NT thickness increased the risk for chromosomal abnormality(11). Several studies have demonstrated high association between absence of nasal bone (NB) observed in the gestational period between 11 and 13 weeks and six days, and trisomy 21(12–14). A recent study has demonstrated that the association between NB evaluation, DV flow pattern and NT thickness in fetuses with high risk for chromosomal abnormalities presents a potential to increase in 2% to 4% the sensitivity of the screening for trisomy 21, or to decrease the false–positive rate in 50%(15). The present study is aimed at evaluating the significance of MA> 35 years, NT thickness, NB evaluation and DV flow pattern in a population of fetuses with high risk for chromosomal abnormalities in the period between 12 and 14 gestational weeks for determining which test or tests combination presents the highest association with chromosomal abnormalities.
MATERIALS AND METHODS The present prospective, observational study was developed during the period between January 2002 and September 2004, with 92 pregnant women between their 12 and 14 gestational weeks, who had been referred to the institution for fetal karyotype study. All of these patients agreed to participate in the present study whose previous approval was granted by the Committee for Ethics in Research of the Institution. The procedures were performed in the Clínica Dr. Antonio Carlos Vieira Lopes, recognized as a reference in fetal medicine in the state of Bahia, and in the Maternidade Climério de Oliveira da Universidade Federal da Bahia (UFBA). The chorionic villus samplings for karyotype study were performed by a single investigator by means of transabdominal chorionic villus biopsy. The banding G technique and polymerase chain reaction for chromosomes 13, 16, 18, 21, 22, X and Y(17) were utilized in the cytogenetic analysis(16). Criteria adopted for indication of chorionic villus sampling were increase in the NT thickness (risk > 1.300) or MA > 35 years. The abdominal approach was adopted in the sonographic studies performed in an Aloka model SSD–2000 equipment coupled with a convex, multifrequential transducer (3.5–5.0 MHz) by two experienced sonographists for evaluating markers of chromosomopathies at the first trimester of gestation. The following sonographic views were utilized for evaluation of the NT thickness: sagittal view of the fetus, the same utilized for the crown–rump length between 45 mm and 84 mm; fetus in neutral position; fetal image occupying at least 75% of the visible screen, calipers placed within the anechoic space between the fetal skin and the subcutaneous tissue overlying the cervical spine. Three measurements were performed for each of the fetuses, and the highest value was considered for the purposes of the present study. Initially, color Doppler was utilized for determining the localization of the vessel, in the DV flow pattern evaluation. The DV flow velocity waveforms were obtained on a mid–sagittal plane of the fetal trunk at < 30° insonation angle, immediately distal to the portal sinus and proximal to the infundibulum of the inferior vena cava. Such a technique avoids the contamination of the flow originating from the intrahepatic portion of the umbilical vein, left hepatic vein and inferior vena cava. The DV flow waveforms were classified as normal (positive flow) or abnormal (absent or reverse flow), according to the blood flow patterns during the atrial contraction period. In the NB evaluation, a mid–sagittal view of the fetal face was acquired, with the fetal dorsum posteriorly positioned and a slight head flexion. Two echogenic lines of the fetal nose profile should be visualized: the superficial echogenic line corresponding to the fetal skin, and the deeper echogenic line corresponding to the NB. The result of a technically satisfactory study where the NB is visualized is classified as present NB. If the NB cannot be visualized, the result is classified as absent NB. Data were recorded on an Excel worksheet (Microsoft, Windows XP) and analyzed by means of a statistical software (SPSS 9.0 for Windows – SPSS Inc., Chicago, Ill., USA). For continuous variables, mean and standard deviation were calculated, and for categorical variables, the percentage was calculated. Aiming at determining the diagnostic accuracy of the tests, sensitivity, specificity, positive predictive value and negative predictive value were calculated, with 95% confidence interval (95% CI). The diagnostic tests were evaluated alone, sequentially and in parallel.
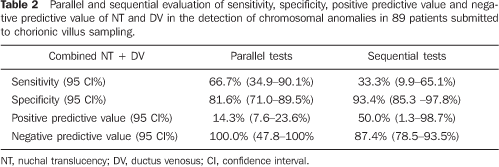
RESULTS Cellular growth was not observed in three of the 92 pregnant women submitted to chorionic villus biopsy. These three cases were excluded from the study. In the remaining 89 pregnant women evaluated for fetal karyotype, chromosomal abnormality was found in 12 fetuses (13.5%), seven of them (58.3%) with trisomy 21, one (8.3%) with trisomy 18, one (8.3%) with trisomy 22, two (16.6%) with 47 XXY, and one (8.3%) with chromosomal marker 46 XY inv (9) (gh). MA ranged between 25 and 47 years (mean = 36.2 years – standard deviation = 4.6).Of the 89 pregnant women, 67 (75.3%) presented ages = 35 years, and 22 (24.7%) presented ages < 35 years. The gestational age at the time of the chorionic villus sampling ranged between 12 and 14 weeks (mean = 13 weeks – standard deviation = 0.79). The main indication for chorionic villus sampling was MA > 35 years in 72 pregnant women (78.3%), followed by high risk for trisomy 21 (> 1:300); based on the NT thickness in 16 pregnant women (17.4%); other reasons, such as previous history of children with genetic diseases; previous history of malformations in children, findings of fetal morphological alterations at ultrasound studies, represented 4.3% of the indications for chorionic villus sampling. NT measurement could be performed in all of the cases, only by transabdominal approach. DV flow evaluation also could be performed in all of the cases. The altered flow ratio (absent or reverse waveform A) corresponded to 6:77 normal fetuses (7.8%). In the group of fetuses with chromosomopathies, this ratio was 5:12 fetuses (41.7%). The evaluation of the NB could be performed in all of the cases, with the NB being present in the whole sample, both in normal fetuses and those with chromosomal alterations. Table 1 shows the sensitivity, specificity, positive predictive value and negative predictive value of NT, DV and MA alone in the detection of chromosomal abnormalities. Table 2 shows a parallel and sequential evaluation of sensitivity, specificity, positive predictive value and negative predictive value of combined NT/DV in the detection of chromosomal abnormalities.
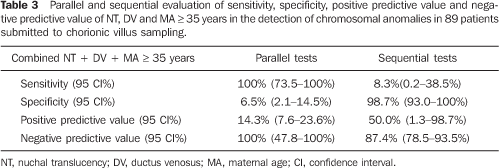
Table 3 shows a parallel and sequential evaluation of sensitivity, specificity, positive predictive value and negative predictive value of combined NT + DV + MA in the detection of chromosomal abnormalities.
DISCUSSION The risk for many chromosomal abnormalities increases with the MA, however, considering that fetuses with chromosomal abnormalities present more frequent intrauterine death, the risk decreases with the gestational age progression(4,5,18). The MA has been the first marker adopted as a method of screening for trisomy 21, with 30% detection rate, and 5% rate of false–positive results(19). Snijders et al.(4) have evaluated 57614 pregnant women with > 35 years of age, between 9 and 16 weeks of gestation, and reported 538 fetuses with trisomy 21. They have observed that the risk for trisomy 21 increases with the MA and decreases with the gestational age. The trisomy 21 prevalence in the period between 12 and 16 gestational weeks was of 30%, whereas with 40 weeks the prevalence decreases to 21%. In the present study, the utilization of the MA alone as a diagnostic test for chromosomal abnormalities presented low sensitivity (58.33%), with low positive predictive value (10.45%), demonstrating its low significance in the screening, when utilized alone. NT has been firstly described by Nicolaides et al.(6) as the screening for fluid collection in the fetal nucha. It is important to note that this accumulation of fluid found in the period between 11 and 14 gestational weeks is present in 75% of fetuses with trisomies 18 and 21. Later, several prospective studies have evidenced the association between the increase in the NT thickness and trisomy 21, with detection rates ranging between 58.3% and 100%(20–23), and false–positive rates around 5%. NT, likewise MA, presented a mild sensitivity (58.33%) and low positive predictive value (35%). A possible explanation for the low sensitivity might be the small number of cases with chromosomal abnormalities evaluated, a result compatible with most of preliminary studies(24–26). Utilizing > 3 mm as cutoff value for NT thickness, the rate of detection for chromosomal abnormalities ranged from 17.6% to 48.4%(24–26). Another study has demonstrated that the inclusion of the DV flow pattern in combination with MA and NT thickness, between 10 and 16 gestational weeks, may reduce the false positive rate in the screening for chromosomal abnormalities(27). In the present study, the utilization of DV flow pattern alone presented a sensitivity of only 41.67% in the detection of chromosomal abnormalities. In the literature, no consensus is found regarding the significance of the utilization of DV flow pattern in the detection of chromosomal abnormalities at the first trimester of gestation, with sensitivity ranging between 65% and 93,1%(10,27). Antolín et al.(27) have evaluated 1371 low–risk pregnant women in the period between 10 and 16 gestational weeks, utilizing the NT thickness and DV flow pattern in the screening for chromosomal abnormalities. DV flow pattern alone presented a global detection rate of 65%, with a false–positive rate of 4.3%. Murta et al.(10) have evaluated 372 low–risk 10–14–week–old fetuses, finding 29 with chromosomal abnormalities. DV flow pattern was altered (zero or reverse waveform A) in 93.1% of fetuses with chromosomal abnormalities and in only 1.7% of normal fetuses. As regards the NB evaluation, several studies have demonstrated the association between absence of the NB at the first gestational trimester with increase in the trisomy 21 detection rate (range = 60%–72.9%), with a false–positive rate of 5%(13, 28,29). Differently from these studies, in the present casuistic, NB evaluation demonstrates low sensitivity for detection of chromosomal abnormalities, considering that the NB was present in all of the cases with chromosomopathies. The results of the present study are compatible with those reported by Malone et al.(30), who have obtained a sensitivity of only 7.7% for NB absence for detecting trisomy 21, i.e., the NB was described as present in nine of 11 fetuses with trisomy 21 (82%). A possible explanation for the lack of sensitivity might be the small number of cases with chromosomal abnormalities, with only seven fetuses with trisomy 21; moreover, incidence of abnormality in NB is variable according to race and ethnics(31). Therefore, it would be premature to conclude that the NB absence is a weak marker for detecting trisomy 21 at the first trimester of gestation.
CONCLUSION Based on the results of the present study, NT thickness, DV flow pattern, NB and MA individually utilized do not demonstrate enough sensitivity to be utilized in the screening for chromosomopathies at the first trimester of gestation. Parallel evaluation of NT thickness and DV flow pattern, one of them with positive results, increases the sensitivity to 66.7% for detecting chromosomal abnormalities, with 14.29% positive predictive value. The major benefit from this association occurs when both results are negative in a patient, considering that, in this case, there is a very high probability (100%) that fetus does not present any chromosomal abnormality. Parallel evaluation of NT thickness, DV flow pattern and MA presented the highest sensitivity (100%), however with a low positive predictive value. According to these results, the best option for screening for chromosomopathies at the fist gestational trimester would be the parallel combination of NT thickness, DV flow pattern and MA. In cases of MA > 35 years, NT thickness and DV flow pattern would be evaluated. Should these results be altered, there would be a significant evidence suggesting the necessity for an invasive investigation for fetal karyotyping. In case of alteration in only one of these results, the couple should be offered an invasive investigation for confirming the fetal karyotype, considering that, individually, the false–positive rate for NT thickness is high (16.88%) and the false–positive rate for DV flow pattern is 8%.
REFERENCES 1. Langdon Down J. Observations on an ethnic classification of idiots. Clinical and Reports London Hospital. 1866;3:259–62. [ ] 2. Smidt–Jensen S, Permin M, Philip J, et al. Randomised comparison of amniocentesis and transabdominal and transcervical chorionic villus sampling. Lancet. 1992;340:1237–44. [ ] 3. Nicolaides K, Brizot Mde L, Patel F, et al. Comparison of chorionic villus sampling and amniocentesis for fetal karyotyping at 10–13 weeks' gestation. Lancet. 1994;344:435–9. [ ] 4. Snijders RJM, Sundberg K, Holzgreve W, et al. Maternal age and gestation–specific risk for trisomy 21. Ultrasound Obstet Gynecol. 1999;13: 167–70. [ ] 5. Snijders RJM, Sebire NJ, Nicolaides KH. Maternal age and gestational age–specific risks for chromosomal defects. Fetal Diagn Ther. 1995;10: 356–67. [ ] 6. Nicolaides KH, Azar G, Snijders RJ, et al. Fetal nuchal oedema: associated malformations and chromosomal defects. Fetal Diagn Ther. 1992;7: 123–31. [ ] 7. Pandya PP, Snijders RJ, Johnson SP, et al. Screening for fetal trisomies by maternal age and fetal nuchal translucency thickness at 10 to 14 weeks of gestation. Br J Obstet Gynaecol. 1995;102:957–62. [ ] 8. Pandya PP, Goldberg H, Walton B, et al. The implementation of the first trimester scanning at 10–13 weeks' gestation and the measurement of fetal nuchal translucency thickness in two maternity units. Ultrasound Obstet Gynecol. 1995;5:20–5. [ ] 9. Montenegro N, Matias A, Areias JC, et al. Increased fetal nuchal translucency: possible involvement of early cardiac failure. Ultrasound Obstet Gynecol. 1997;10:265–8. [ ] 10. Murta CG, Moron AF, Avila MA, et al. Application of ductus venosus Doppler velocimetry for the detection of fetal aneuploidy in the first trimester of pregnancy. Fetal Diagn Ther. 2002;17: 308–14. [ ] 11. Zoppi MA, Putzolu M, Ibba RM, et al. First–trimester ductus venosus velocimetry in relation to nuchal translucency thickness and fetal karyotype. Fetal Diagn Ther. 2002;17:52–7. [ ] 12. Cicero S, Curcio P, Papageorghiou A, et al. Absence of nasal bone in fetuses with trisomy 21 at 11–14 weeks of gestation: an observational study. Lancet. 2001;358:1665–7. [ ] 13. Otano L, Aiello H, Igarzábal L, et al. Association between first trimester absence of fetal nasal bone on ultrasound and Down syndrome. Prenat Diagn. 2002;22:930–2. [ ] 14. Zoppi MA, Ibba RM, Axiana C, et al. Absence of fetal nasal bone and aneuploidies at first–trimester nuchal translucency screening in unselected pregnancies. Prenat Diagn. 2003;23:496–500. [ ] 15. Prefumo F, Sethna F, Sairam S, et al. First–trimester ductus venosus, nasal bones, and Down syndrome in a high–risk population. Obstet Gynecol. 2005;105:1348–54. [ ] 16. Sanchez O, Escobar JI, Yunis JJ. A simple G–banding technique. Lancet 1973;2:269–71. [ ] 17. van den Berg C, Van Opstal D, Brandenburg H, et al. Accuracy of abnormal karyotypes after the analysis of both short– and long–term culture of chorionic villi. Prenat Diagn. 2000;20:956–69. [ ] 18. Snijders RJM, Holzgreve W, Cuckle H, et al. Maternal age–specific risks for trisomies at 9–14 weeks' gestation. Prenat Diagn. 1994;14:543–52. [ ] 19. Cuckle H. Integrating antenatal Down's syndrome screening. Curr Opin Obstet Gynecol. 2001;13: 175–81. [ ] 20. Audibert F, Dommergues M, Benattar C, et al. Screening for Down syndrome using first–trimester ultrasound and second–trimester maternal serum markers in a low–risk population: a prospective longitudinal study. Ultrasound Obstet Gynecol. 2001;18:26–31. [ ] 21. Chasen ST, Sharma G, Kalish RB, et al. First–trimester screening for aneuploidy with fetal nuchal translucency in a United States population. Ultrasound Obstet Gynecol. 2003;22:149–51. [ ] 22. Zoppi MA, Ibba RM, Floris M, et al. Fetal nuchal translucency screening in 12495 pregnancies in Sardinia. Ultrasound Obstet Gynecol. 2001;18: 649–51. [ ] 23. Wayda K, Keresztúri A, Orvos H, et al. Four years experience of first–trimester nuchal translucency screening for fetal aneuploidies with increasing regional availability. Acta Obstet Gynecol Scand. 2001;80:1104–9. [ ] 24. Comas C, Martinez JM, Ojuel J, et al. First–trimester nuchal edema as a marker of aneuploidy. Ultrasound Obstet Gynecol. 1995;5:26–9. [ ] 25. Ville Y, Lalondrelle C, Doumerc S, et al. First–trimester diagnosis of nuchal anomalies: significance and fetal outcome. Ultrasound Obstet Gynecol. 1992;2:314–6. [ ] 26. Trauffer PM, Anderson CE, Johnson A, et al. The natural history of euploid pregnancies with first–trimester cystic hygromas. Am J Obstet Gynecol. 1994;170:1279–84. [ ] 27. Antolín E, Comas C, Torrents M, et al. The role of ductus venosus blood flow assessment in screening for chromosomal abnormalities at 10–16 weeks of gestation. Ultrasound Obstet Gynecol. 2001;17:295–300. [ ] 28. Wong SF, Choi H, Ho LC. Nasal bone hipoplasia: is it a common finding amongst chromosomally normal fetuses of southern Chinese women? Gynecol Obstet Invest. 2003;56:99–101. [ ] 29. Cicero S, Sonek JD, McKenna DS, et al. Nasal bone hipoplasia in trisomy 21 at 15–22 weeks' gestation. Ultrasound Obstet Gynecol. 2003;21: 15–8. [ ] 30. Malone FD, Ball RH, Nyberg DA, et al. First–trimester nasal bone evaluation for aneuploidy in the general population. Obstet Gynecol. 2004; 104:1222–8. [ ] 31. Collado F, Bombard A, Li V, et al. Ethnic variation of fetal nasal bone length between 11–14 weeks' gestation. Prenat Diagn. 2005;25:690–2. [ ] Received June 18, 2007. Accepted after revision July 24, 2007. * Study developed at Faculdade de Medicina da Universidade Federal da Bahia (UFBA), Salvador, BA, Brazil. |
|
Av. Paulista, 37 - 7° andar - Conj. 71 - CEP 01311-902 - São Paulo - SP - Brazil - Phone: (11) 3372-4544 - Fax: (11) 3372-4554