Radiologia Brasileira - Publicação Científica Oficial do Colégio Brasileiro de Radiologia
AMB - Associação Médica Brasileira CNA - Comissão Nacional de Acreditação
 Vol. 41 nº 2 - Mar. / Apr. of 2008
Vol. 41 nº 2 - Mar. / Apr. of 2008
|
ORIGINAL ARTICLE
|
|
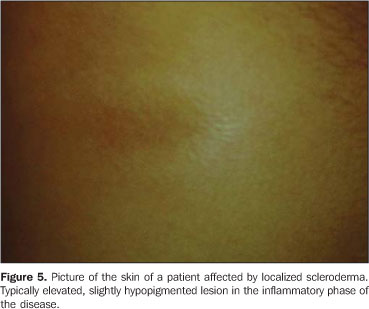
Clinical and ultrasonographic correlation in localized cutaneous scleroderma |
|
|
Autho(rs): Marcio Bouer, Maria Cristina Chammas, Maria Cristina Lorenzo Messina, Ilka Regina Souza de Oliveira, Giovanni Guido Cerri |
|
|
Keywords: Localized scleroderma, Ultrasonography, Dermis |
|
|
Abstract:
IMD, Radiologist, Researcher for the Unit of Ultrasonography, Instituto de Radiologia – Hospital das Clínicas da Faculdade de Medicina da Universidade de São Paulo (InRad/HC-FMUSP), São Paulo, SP, Brazil
INTRODUCTION Scleroderma is a disease of unknown etiology affecting the conjunctive tissue and characterized by sclerotic alteration of the skin and some other organ systems. Histological findings on the skin are: massive deposition of synthesized collagen, perivascular mononuclear cell infiltrate and vascular damages(1). Scleroderma was first studied by means of ultrasonography in 1984, with a 15 MHz transducer (A-mode). In cases of focal lesions, the skin thickness was increased, and in pigmented lesions, decreased(2). In 1986, and later in 1993, B-mode 10 MHz, 20 MHz and 25 MHz transducers started being utilized, and could demonstrate increase in the skin thickness in the involved regions, as well as post-treatment alterations(3–5). In 1995, a study with a 30 MHz transducer demonstrated an increase in the skin thickness of involved regions and even a mild increase in clinically non-affected regions(6). In 1998, post-treatment imaging of the skin with a 20 MHz transducer (B-mode) demonstrated that there may be different findings depending on the disease stage(7). The present study was aimed at demonstrating ultrasonographic findings as compared with clinical findings in cases of focal (or localized) scleroderma.
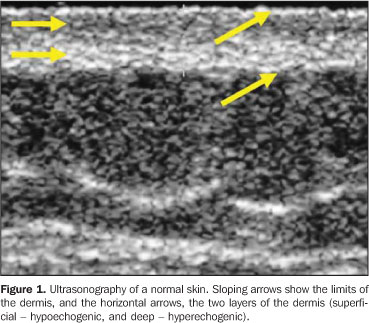
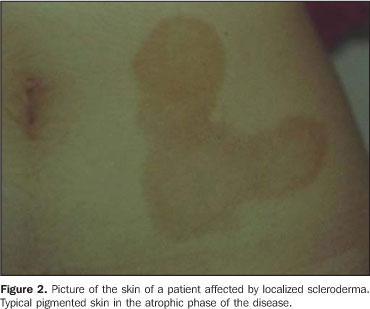
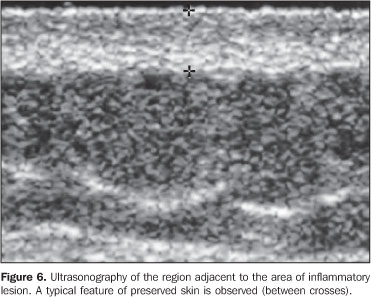
MATERIALS AND METHODS Twenty-three lesions of focal scleroderma were evaluated in 21 patients (18 women and 3 men) with ages ranging between 8 and 58 years (mean age, 24.1 years), in the period between May 2001 and May 2003, with a Logiq 700 (GE Medical Systems; Milwaukee, Wis., USA) equipment coupled with a multifrequency linear transducer (6–14 MHz). Lesions were found on the trunk (15) and limbs (8) of these patients. The study was submitted to the approval by the Committee for Ethics in Research of Hospital das Clínicas da Faculdade de Medicina da Universidade de São Paulo, and all of the participating patients signed a term of free and informed consent. The studies were performed by an experienced radiologist specialized in small parts ultrasound imaging, with a thick gel layer between the transducer and the skin, and minimum pressure on the region to be scanned for not deforming the surface of the skin to be evaluated. Parameters to be evaluated at B-mode ultrasound included: a) dermis thickness – measured from the anterior hyperechogenic line between the gel layer and the dermis surface, and the posterior hyperechogenic line between the deep dermis and the subcutaneous tissue (Figure 1); b) the dermis echogenicity – normally characterized by a hypoechogenic superficial layer and a hyperechogenic deep layer (Figure 2).
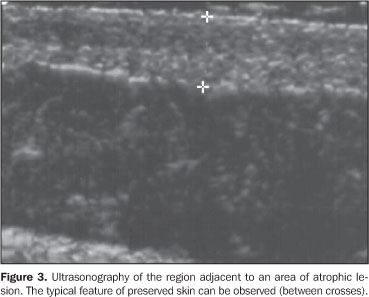
Both the region of the lesion and an adjacent healthy region of the skin were evaluated in all of the patients participating in the present study. Seven patients had a post-treatment follow-up including the evaluation of these same parameters. The clinical diagnosis was made by an experienced dermatologist, and all of the cases had their diagnosis confirmed by means of anatomopathological studies.
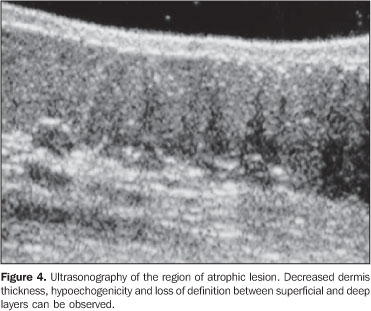
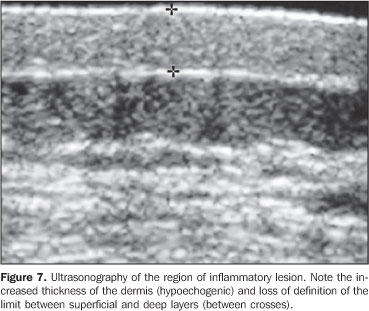
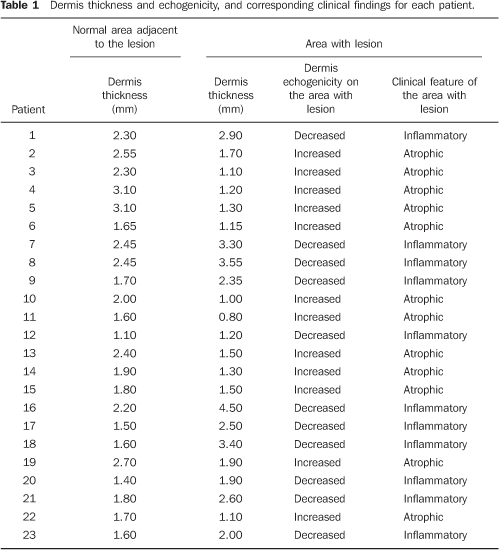
RESULTS In the thickness analysis, clinically atrophic lesions (Figure 2), corresponding to 52.2% (12/23) of cases, presented a decrease in the dermis thickness (Figure 4) as compared with the clinically normal regions (Figure 3). The mean thickness in the affected regions was 1.29 mm, whereas in the normal regions was 2.23 mm. On the other hand, clinically inflammatory lesions, corresponding to 47.8% (11/23) of cases (Figure 5), presented an increase in the dermis thickness (Figure 7) as compared with the clinically normal regions (Figure 6). The mean thickness in the affected regions was 2.90 mm, whereas in the normal regions, it was 1.90 mm (Table 1).
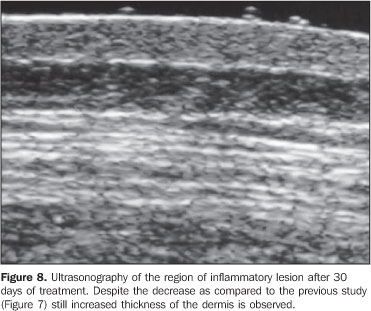
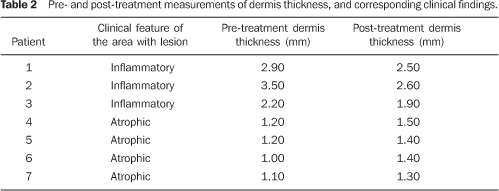
As regards echogenicity, in cases of atrophic lesion, the dermis presented with an increase in echogenicity (Figure 4), whereas on inflammatory lesions the dermis presented with a decrease in echogenicity (Figure 7). Loss of the typical ultrasonographic pattern of the dermis, corresponding to superficial hypoechogenicity and deep hyperechogenicity for all of the affected regions, was observed in all of the affected regions (Figures 3 and 6). The seven patients who underwent post-treatment follow-up, presented with alteration of the dermis thickness. Three of these cases presented inflammatory lesion with decreased dermis thickness (from 2.90 mm to 2.30 mm, on average) (Figure 8), and four presented atrophic lesions, with increased dermis thickness (from 1.10 mm to 1.40 mm, on average) (Table 2).
All anatomopathological studies confirmed the clinical diagnosis of scleroderma, both in the inflammatory phase, with predominance of cell infiltrate, and in the atrophic phase, with massive deposition of synthesized connective tissue and fibrosis.
DISCUSSION The utilization of ultrasonography with high frequency transducers in the evaluation of dermatologic diseases has increased with recent technological developments and the demand for a reliable and non-invasive method to aid the dermatologist in the visualization of abnormalities such as tumors or other less aggressive diseases occurring under the affected skin. In 1979, Alexander & Miller were the first to apply ultrasonography in dermatology to measure the skin thickness(8). From that time on, many other studies about the most different dermatological diseases have been developed, utilizing already available equipment/transducers as well as other devices especially designed for this purpose. The normal skin is made up of three different layers: a) epidermis, a 0.06–0.6 mm-thick layer with two types of cells – keratinocytes and melanocytes; b) dermis, a 1–4 mm-thick layer constituted of an extensive network of blood and lymphatic vessels, connective tissue, nerves, glands, fibroblasts, histiocytes and mastocytes, divided into papillary dermis – more superficial and thinner, with loose connective tissue, and reticular dermis, deeper and with a dense connective tissue; c) subcutaneous tissue, with a variable thickness, consisting predominantly of fat cells. The image of a normal skin, from the surface to the inner layers, will show:
Scleroderma physiopathology is complex and consists in three main factors: vascular damage, mononuclear cell infiltrate, and massive deposition of newly synthesized connective tissue, remarkably collagen. Apparently, these three factors occur simultaneously, initially with predominance of cellular infiltrate, with an increase in the dermis thickness corresponding to the inflammatory phase of the disease, and latter predominance of deposition of connective tissue and fibrosis, with decrease in the dermis thickness, corresponding to the atrophic phase of the disease(1). The ultrasonographic findings are compatible with the histological findings: clinically atrophic lesions presented a decrease in the dermis thickness, and clinically inflammatory lesions showed an increase in the dermis thickness. The studies developed by Serup(2), Akesson et al.(3), Myers et al.(4) and Ihn et al.(6) have reported increase in the thickness of the dermis in the regions affected by scleroderma, probably because the inflammatory phase predominated in the cases evaluated. Atrophic lesions presented an increase in the dermis echogenicity, whereas inflammatory lesions presented a decrease in the echogenicity. Additionally, in the affected areas, there was a loss of differentiation between the superficial and deep layers of the dermis which has not been described in other studies and that can be explained by the diffuse involvement of the reticular dermis; in the case of edematous predominance, by the presence of cellular infiltrate and inflammatory process; and in the atrophic case, by fibrosis and higher collagen concentration. During the treatment, alterations were observed in the dermis thickness, with inflammatory lesions presenting a decrease in the thickness of the dermis, and atrophic lesions presenting an increase, however, the differentiation between the superficial and deep layers of the dermis could not be established. Other authors(3,4,7,10,11) have concluded that ultrasonography is useful for a later posttreatment follow-up. Finally, ultrasonographic findings allow the association between the increase in the dermis thickness/ decrease in the dermis echogenicity and the inflammatory phase of the disease, where edema and inflammatory process predominate, and the association between the decrease in the dermis thickness/increase in the dermis echogenicity and the atrophic phase of the disease, where loss of the skin elasticity and dermis cells compaction may be observed. Also, one can observe that it is possible to quantify the dermis thickness, utilizing this information in the post-treatment follow-up to indicate, or not, an improvement associated with the clinical evaluation. The development of transducers with higher frequencies as well as devices with higher resolution can improve the post-treatment evaluation of the dermis thickness, and also de detection of alterations on clinically normal regions.
REFERENCES 1. Sapadin AN, Esser AC, Fleischmajer R. Immunopathogenesis of scleroderma – evolving concepts. Mt Sinai J Med. 2001;68:233–42. [ ] 2. Serup J. Localized scleroderma (morphoea): thickness of sclerotic plaques as measured by 15 MHz pulsed ultrasound. Acta Derm Venereol. 1984;64: 214–9. [ ] 3. Akesson A, Forsberg L, Hederstrom E, et al. Ultrasound examination of skin thickness in patients with progressive systemic sclerosis (scleroderma). Acta Radiol Diagn. 1986;27:91–4. [ ] 4. Myers SL, Cohen JS, Sheets PW, et al. B–mode ultrasound evaluation of skin thickness in progressive systemic sclerosis. J Rheumatol. 1986; 13:577–80. [ ] 5. Fornage BD, McGavran MH, Duvic M, et al. Imaging of the skin with 20–MHz US. Radiology. 1993;189:69–76. [ ] 6. Ihn H, Shimozuma M, Fujimoto M, et al. Ultrasound measurement of skin thickness in systemic sclerosis. Br J Rheumatol. 1995;34:535–8. [ ] 7. Cammarota T, Pinto F, Magliaro A, et al. Current uses of diagnostic high–frequency US in dermatology. Eur J Radiol. 1998;27:S215–23. [ ] 8. Alexander H, Miller DL. Determining skin thickness with pulsed ultrasound. J Invest Dermatol. 1979;72:17–9. [ ] 9. Bouer M, Saito OC. Pele, subcutâneo e parede. In: Saito OC, Cerri GG, editores. Ultra–sonografia de pequenas partes. São Paulo: Revinter; 2004. p. 245–68. [ ] 10. Hoffmann K, Gerbaulet U, el–Gammal S, et al. 20–MHz B–mode ultrasound in monitoring the course of localized scleroderma (morphea). Acta Derm Venereol Suppl. 1991;164:3–16. [ ] 11. Scheja A, Akesson A. Comparison of high frequency (20 MHz) ultrasound and palpation for the assessment of skin involvement in systemic sclerosis (scleroderma). Clin Exp Rheumatol. 1997;15:283–8. [ ] Received May 22, 2007. Accepted after revision July 24, 2007. * Study developed at Instituto de Radiologia – Hospital das Clínicas da Faculdade de Medicina da Universidade de São Paulo (InRad/HC-FMUSP), São Paulo, SP, Brazil. |
|
Av. Paulista, 37 - 7° andar - Conj. 71 - CEP 01311-902 - São Paulo - SP - Brazil - Phone: (11) 3372-4544 - Fax: (11) 3372-4554