Radiologia Brasileira - Publicação Científica Oficial do Colégio Brasileiro de Radiologia
AMB - Associação Médica Brasileira CNA - Comissão Nacional de Acreditação
 Vol. 41 nº 2 - Mar. / Apr. of 2008
Vol. 41 nº 2 - Mar. / Apr. of 2008
|
WHICH IS YOUR DIAGNOSIS?
|
|
Which is your diagnosis? |
|
|
Autho(rs): Marcelo Souto Nacif, Antonio Carlos Pires Carvalho, Alair Augusto Sarmet Moreira Damas dos Santos, Telmo Pimentel do Vabo, Carlos Eduardo Rochitte, Edson Marchiori |
|
|
IFellow PhD degree in Radiology (Cardiac MRI), Universidade Federal do Rio de Janeiro (UFRJ), Rio de Janeiro, RJ, Responsible for the Department of Cardiac Magnetic Resonance Imaging at Hospital de Clínicas de Niterói (HCN), Niterói, RJ, Brazil
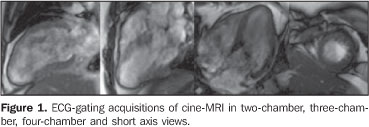
A female, 43-year-old patient weighting 95 kg, with 152 cm in height, cardiac frequency of 80 bpm, blood pressure of 100 × 70 mmHg, presenting with atypical precordial pain and increase in troponin levels. The patient reported diarrhea 72 hours previously to the episode. The initial suspicion of myocarditis was considered. The patient was referred to the Division of Radiology and Imaging Diagnosis at Hospital de Clínicas de Niterói, for cardiac magnetic resonance imaging (CMRI). Images description Figure 1. ECG-gating acquisitions of cine-MRI in two-chamber, three-chamber, four-chamber and short axis views demonstrate global systolic dysfunction, with estimated ejection fraction of 41% (Simpson), middle and apical hypokinesia with thinning of the apex and apical akinesis. Estimated left ventricular mass was 125 grams (± 11).
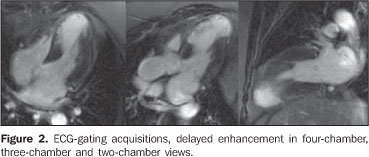
Figure 2. ECG-gating acquisitions, delayed enhancement in four-chamber, three-chamber and two-chamber views demonstrate the delayed myocardial enhancement, transmural enhancement (> 70% of the segmental area) in the medial and apical antero-septal segments, in the septal and lower apical segments, and in the apex, characterizing the presence of infarct. There is a delayed myocardial, non-transmural enhancement (< 50% of the segmental area) in the mid-infero-septal and latero-apical segments.
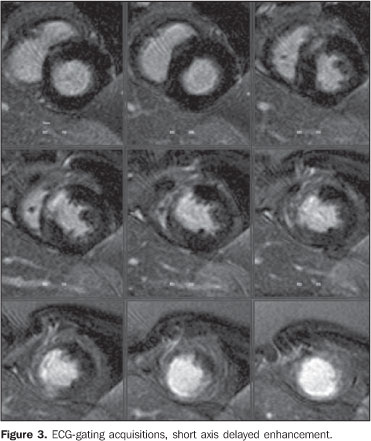
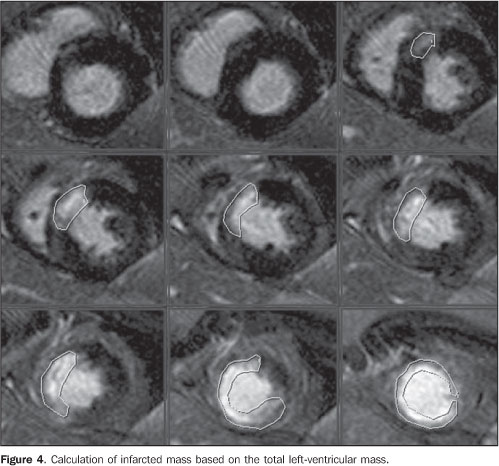
Figure 3. ECG-gating acquisitions, shot axis delayed enhancement more clearly demonstrating the myocardial segmentation and delayed enhancement in the region of the anterior descending coronary. The segmental infarcted mass weights approximately 30 grams, with the percentage of infarcted mass corresponding to 22%.
Diagnosis: Transmural myocardial infarction, representing 22% of the left ventricular mass.
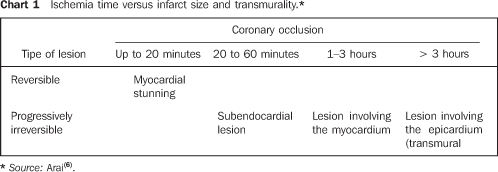
COMMENTS Heart disease and stroke are the leading causes of death or disability in the United States, significantly contributing to the increase in public health costs in this country. Coronary artery disease accounts for the highest rate of heart diseases affecting as many 12 million inhabitants in the United States(1,2). According to Datasus, in Brazil, cardiovascular diseases account for up to 30% of deaths, with a 22% increase in prevalence in the last 19 years(3). Acute myocardial infarction is the rapid development of myocardial necrosis because of an imbalance between the oxygen supply and the cardiac muscle demand. A total coronary occlusion for a four-to-six-hour period results in irreversible myocardial necrosis (Chart 1). Reperfusion within this period can salvage the myocardium, reducing morbidity and mortality(1,2,4–6).
The most frequent cause of acute myocardial infarction is the narrowing of the epicardial arterial vessels due to atheromatous plaques. Plaque rupture with subsequent exposure of the subendothelial tissue results in platelet aggregation, formation of thrombi, fibrin accumulation and vasospasm. This may result in a partial or complete occlusion of the vessel, ischemia and consequential myocardial infarction(3,5,6). Myocardial viability The myocardial viability testing has shown to be a quite useful method for assessing patients with coronary artery disease and severe left ventricular dysfunction, considering that the conduct to be adopted — revascularization/angioplasty (with or without stent) or clinical treatment — is directly influenced by the presence or not of viable tissue. The term "viable myocardium" refers to myocardial tissue with regional dysfunction potentially reversible after revascularization, and covers different tissue subtypes: hibernating myocardium and stunned myocardium (Chart 2)(6). Hibernating myocardium results from chronic and persistent ischemia, where the oxygen consumption is decreased resulting in ventricular dysfunction. Stunned myocardium occurs after an acute and short episode of severe ischemia, resulting in ultra-structural and biochemical alterations with consequential ventricular dysfunction. Coronary revascularization in patients with myocardial viability has improved the left ventricular wall contractility, ejection fraction, functional classification and prognosis. The major challenge is to determine which patients will benefit from the intervention, hence the significance of the myocardial viability testing(6–8). Cardiac magnetic resonance imaging CMRI is highly accurate in the detection of myocardial necrosis, which in some cases is subdiagnosed by clinical and electrocardiographic data(7–9). Besides functional and contractility alterations identified on cine-MRI studies, an inversion recovery sequence with high resolution performed about ten minutes after intravenous gadolinium injection is the main technique utilized in the diagnosis of myocardial infarction and is currently known as delayed myocardial enhancement technique(10–13). This method is based on the characteristics of gadolinium distribution throughout the different tissues such as the normal myocardium, the recently infarcted myocardium and fibrotic myocardium. Gadolinium is a contrast agent specific for extracellular spaces imaging, that is to say, it does not diffuse within normal cells. So, a normal myocardium presents less extracellular space than infarction and fibrosis, considering that, in these tissues, the myocyte membrane rupture connects the intra- and extracellular compartments(9–11,13). Delayed myocardial enhancement allows an accurate delimitation of necrotic or fibrotic areas in the myocardium of patients with previous infarct. On delayed-enhanced images, infarct areas present high signal intensity as compared with the normal myocardium. The high contrast between fibrotic or necrotic tissue and intact myocardial tissue where gadolinium does not remain for a long time represents about ten-fold the signal intensity, allowing an accurate evaluation of the infarcted territory(10–13). Additionally to myocyte injury (necrosis/infarct), destruction of the microcirculation may occur, depending on the time of myocardial exposure to low or absent perfusion, and, even in the case of segmental reperfusion, the injured tissue cannot be perfunded again, determining the severity of the lesion. These are the no-reflow zones or, more precisely, regions of microvascular obstructions which can be identified as hypoenhanced areas within the infarct, reaffirming the non-viability of that coronary segment territory. This is due to the absence of gadolinium in these areas(4,6,9,10). The study developed by Wu et al.(10) in 1998 demonstrated that the infarct size, expressed as a percentage of the left ventricular mass, has a significant prognostic value for patients with acute myocardial infarct. Additionally, other two recent studies have demonstrated that the assessment of the infarcted mass was a predictor of recovery of the segmental and global systolic function(9,10). The evaluation of myocardial viability by MRI has made a high impact in the literature, particularly after the study developed by Kim et al.(11), demonstrating that the presence of myocardial viability, defined as regional functional recovery following revascularization, can be determined by the quantification of infarct transmurality. This same study has also demonstrated that the greater the dysfunctional myocardial mass viable prior to the intervention, the greater the overall recovery of the ejection fraction following the revascularization(11–13). Figure 4 demonstrates the measurement of the infarcted mass analyzed in conjunction with the total left ventricular mass at diastole, resulting in a percentage of infarcted mass which, in the present study corresponded to 22%.
Therefore, the determination of the infarcted mass by means of CMRI can provide significant and extremely useful information for the management of patients with previous acute myocardial infarction. Final consideration Currently, CMRI is considered as the method of choice in the detection of myocardial infarction, viability, and evaluation of left ventricular infarcted mass, surpassing PET-CT in the detection of subendocardial defects. Improvement in contractility and regional thickening, level of heart failure, increase in survival and improvement in the patient´s quality of life after procedures of myocardial reperfusion justify the increasing interest in the utilization of cardiac MRI for detecting myocardial viability.
REFERENCES 1. Rochitte CE, Pinto IMF, Fernandes JL, et al. I Diretriz de ressonância e tomografia cardiovascular da Sociedade Brasileira de Cardiologia. Arq Bras Cardiol. 2006;87:e48–59. [ ] 2. Azevedo Filho CF, Hadlich M, Petriz JLF, et al. Quantification of left ventricular infarcted mass on cardiac magnetic resonance imaging. Comparison between planimetry and the semiquantitative visual scoring method. Arq Bras Cardiol. 2004;83:111–7. [ ] 3. Dados do Datasus. [Acessado em 22 de janeiro de 2007]. Disponível em: http://portal.saude.gov. br/portal/aplicacoes/noticias/noticias_detalhe. cfm?co_seq_noticias=38183 [ ] 4. Rochitte CE, Lima JA, Bluemke DA, et al. Magnitude and time course of microvascular obstruction and tissue injury after acute myocardial infarction. Circulation. 1998;98:1006–14. [ ] 5. Kim RJ, Wu E, Rafael A, et al. The use of contrast–enhanced magnetic resonance imaging to identify reversible myocardial dysfunction. N Engl J Med. 2000;343:1445–53. [ ] 6. Arai AE. Myocardial infarction and viability with an emphasis on imaging delayed enhancement. In: Kwong RY, editor. Cardiovascular magnetic resonance imaging. Totowa: Humana Press; 2008. p. 351–75. [ ] 7. Simonetti OP, Kim RJ, Fieno DS, et al. An improved MR imaging technique for the visualization of myocardial infarction. Radiology. 2001; 218:215–23. [ ] 8. Mahrholdt H, Wagner A, Holly TA, et al. Reproducibility of chronic infarct size measurement by contrast–enhanced magnetic resonance imaging. Circulation. 2002;106:2322–7. [ ] 9. Foo TK, Stanley DW, Castillo E, et al. Myocardial viability: breath–hold 3D MR imaging of delayed hyperenhancement with variable sampling in time. Radiology. 2004;230:845–51. [ ] 10. Wu KC, Kim RJ, Bluemke DA, et al. Quantification and time course of microvascular obstruction by contrast–enhanced echocardiography and magnetic resonance imaging following acute myocardial infarction and reperfusion. J Am Coll Cardiol. 1998;32:1756–64. [ ] 11. Kim RJ, Chen EL, Lima JA, et al. Myocardial Gd–DTPA kinetics determine MRI contrast enhancement and reflect the extent and severity of myocardial injury after acute reperfused infarction. Circulation. 1996;94:3318–26. [ ] 12. Nacif MS, Santos AASMD, Barros Filho CM, et al. Qual o seu diagnóstico? Radiol Bras. 2007; 40(5):vii–x. [ ] 13. Bezerra LB, Marchiori E, Pontes PV. Avaliação da função cardíaca por ressonância magnética com seqüências em equilíbrio estável: segmentadas × tempo real. Radiol Bras. 2006;39:333–9. [ ] Study developed at Hospital de Clínicas de Niterói (HCN), Niterói, RJ, and Universidade Federal do Rio de Janeiro (UFRJ), Rio de Janeiro, RJ, Brazil. |
|
Av. Paulista, 37 - 7° andar - Conj. 71 - CEP 01311-902 - São Paulo - SP - Brazil - Phone: (11) 3372-4544 - Fax: (11) 3372-4554